Abstract
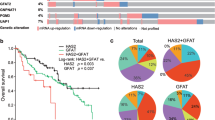
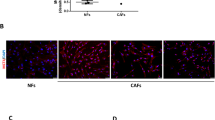
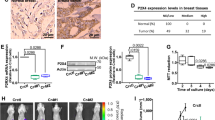
An improved understanding of the biochemical alterations that accompany tumor progression and metastasis is necessary to inform the next generation of diagnostic tools and targeted therapies. Metabolic reprogramming is known to occur during the epithelial–mesenchymal transition (EMT), a process that promotes metastasis. Here, we identify metabolic enzymes involved in extracellular matrix remodeling that are upregulated during EMT and are highly expressed in patients with aggressive mesenchymal-like breast cancer. Activation of EMT significantly increases production of hyaluronic acid, which is enabled by the reprogramming of glucose metabolism. Using genetic and pharmacological approaches, we show that depletion of the hyaluronic acid precursor UDP-glucuronic acid is sufficient to inhibit several mesenchymal-like properties including cellular invasion and colony formation in vitro, as well as tumor growth and metastasis in vivo. We found that depletion of UDP-glucuronic acid altered the expression of PPAR-gamma target genes and increased PPAR-gamma DNA-binding activity. Taken together, our findings indicate that the disruption of EMT-induced metabolic reprogramming affects hyaluronic acid production, as well as associated extracellular matrix remodeling and represents pharmacologically actionable target for the inhibition of aggressive mesenchymal-like breast cancer progression.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Code availability
The source code used is available on https://github.com/SreekumarLab/Publications.
Change history
25 March 2020
A Correction to this paper has been published: https://doi.org/10.1038/s41388-020-1252-1
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30.
Mani SA, Guo W, Liao M-J, Eaton EN, Ayyanan A, Zhou AY, et al. The epithelial–mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–15.
Bhowmik SK, Ramirez-Peña E, Arnold JM, Putluri V, Sphyris N, Michailidis G, et al. EMT-induced metabolite signature identifies poor clinical outcome. Oncotarget 2015;6:42651–60.
Curtis C, Shah SP, Chin S-F, Turashvili G, Rueda OM, Dunning MJ, et al. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature 2012;486:346–52.
Neve RM, Chin K, Fridlyand J, Yeh J, Baehner FL, Fevr T, et al. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell 2006;10:515–27.
Györffy B, Lanczky A, Eklund AC, Denkert C, Budczies J, Li Q, et al. An online survival analysis tool to rapidly assess the effect of 22,277 genes on breast cancer prognosis using microarray data of 1,809 patients. Breast Cancer Res Treat. 2010;123:725–31.
Vigetti D, Viola M, Karousou E, De Luca G, Passi A. Metabolic control of hyaluronan synthases. Matrix Biol. 2014;35:8–13.
Li M, Pathak RR, Lopez-Rivera E, Friedman SL, Aguirre-Ghiso JA, Sikora AG. The in ovo chick chorioallantoic membrane (CAM) assay as an efficient xenograft model of hepatocellular carcinoma. J Vis Exp. 2015;104:52411
Yates TJ, Lopez LE, Lokeshwar SD, Ortiz N, Kallifatidis G, Jordan A, et al. Dietary supplement 4-methylumbelliferone: an effective chemopreventive and therapeutic agent for prostate cancer. J Natl Cancer Inst. 2015;107:djv085
Kultti A, Pasonen-Seppänen S, Jauhiainen M, Rilla KJ, Kärnä R, Pyöriä E, et al. 4-Methylumbelliferone inhibits hyaluronan synthesis by depletion of cellular UDP-glucuronic acid and downregulation of hyaluronan synthase 2 and 3. Exp Cell Res. 2009;315:1914–23.
Oyinlade O, Wei S, Lal B, Laterra J, Zhu H, Goodwin CR, et al. Targeting UDP-α-d-glucose 6-dehydrogenase inhibits glioblastoma growth and migration. Oncogene 2018;37:2615–29.
Misra S, Hascall VC, Markwald RR, Ghatak S. Interactions between hyaluronan and its receptors (CD44, RHAMM) regulate the activities of inflammation and cancer. Front Immunol. 2015;6:201.
Saito T, Dai T, Asano R. The hyaluronan synthesis inhibitor 4-methylumbelliferone exhibits antitumor effects against mesenchymal-like canine mammary tumor cells. Oncol Lett. 2013;5:1068–74.
Sato N, Kohi S, Hirata K, Goggins M. Role of hyaluronan in pancreatic cancer biology and therapy: once again in the spotlight. Cancer Sci. 2016;107:569–75.
Dong C, Yuan T, Wu Y, Wang Y, Fan TWM, Miriyala S, et al. Loss of FBP1 by snail-mediated repression provides metabolic advantages in basal-like breast cancer. Cancer Cell 2013;23:316–31.
Lucena MC, Carvalho-Cruz P, Donadio JL, Oliveira IA, de Queiroz RM, Marinho-Carvalho MM, et al. Epithelial mesenchymal transition induces aberrant glycosylation through hexosamine biosynthetic pathway activation. J Biol Chem. 2016;291:12917–29.
Toole BP. Hyaluronan-CD44 interactions in cancer: paradoxes and possibilities. Clin Cancer Res. 2009;15:7462–8.
Ricciardelli C, Ween MP, Lokman NA, Tan IA, Pyragius CE, Oehler MK. Chemotherapy-induced hyaluronan production: a novel chemoresistance mechanism in ovarian cancer. BMC Cancer 2013;13:476.
Jacobetz MA, Chan DS, Neesse A, Bapiro TE, Cook N, Frese KK, et al. Hyaluronan impairs vascular function and drug delivery in a mouse model of pancreatic cancer. Gut. 2013;62:112–20.
Hollier BG, Tinnirello AA, Werden SJ, Evans KW, Taube JH, Sarkar TR, et al. FOXC2 expression links epithelial–mesenchymal transition and stem cell properties in breast cancer. Cancer Res. 2013;73:1981–92.
Battula VL, Evans KW, Hollier BG, Shi Y, Marini FC, Ayyanan A, et al. Epithelial-mesenchymal transition-derived cells exhibit multilineage differentiation potential similar to mesenchymal stem cells. Stem Cells 2010;28:1435–45.
Putluri N, Shojaie A, Vasu VT, Vareed SK, Nalluri S, Putluri V, et al. Metabolomic profiling reveals potential markers and bioprocesses altered in bladder cancer progression. Cancer Res. 2011;71:7376–86.
Gentleman R. Bioinformatics and computational biology solutions using R and Bioconductor [Internet]. New York: Springer Science+Business Media; 2005 [cited 15 Apr 2019].
Putluri N, Maity S, Kommagani R, Kommangani R, Creighton CJ, Putluri V, et al. Pathway-centric integrative analysis identifies RRM2 as a prognostic marker in breast cancer associated with poor survival and tamoxifen resistance. Neoplasia. 2014;16:390–402.
Dasgupta S, Putluri N, Long W, Zhang B, Wang J, Kaushik AK, et al. Coactivator SRC-2–dependent metabolic reprogramming mediates prostate cancer survival and metastasis. J Clin Investig. 2015;125:1174–88.
Marshall J. Transwell(®) invasion assays. Methods Mol Biol. 2011;769:97–110.
Werden SJ, Sphyris N, Sarkar TR, Paranjape AN, LaBaff AM, Taube JH, et al. Phosphorylation of serine 367 of FOXC2 by p38 regulates ZEB1 and breast cancer metastasis, without impacting primary tumor growth. Oncogene 2016;35:5977–88.
van der Horst EH, Leupold JH, Schubbert R, Ullrich A, Allgayer H. TaqMan-based quantification of invasive cells in the chick embryo metastasis assay. BioTechniques. 2004;37:940–2. 944, 946
Kuleshov MV, Jones MR, Rouillard AD, Fernandez NF, Duan Q, Wang Z, et al. Enrichr: a comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 2016;44:W90–7.
Acknowledgements
Thanks to Mariana Villanueva MS and Ravi Pathak Ph.D. M.B.A, in the Baylor College of Medicine Advanced in vivo Models Core for assistance with chicken chorioallantoic membrane assays. This research was partially funded by National Cancer Institute grant numbers U01CA179674-01A1 (AS), National Science Foundation NSF PHY-1605817 (SAM) NCI R01CA200970 (SAM), P30CA125123 Metabolomics Shared Resources (AS), R01CA220297 (NP), and partially supported by P50CA186784 to C.K. Osborne; CPRIT grant numbers RP170005 to the Proteomics and Metabolomics Core Facility (AS and NP); CPRIT Core Facilities Support Grant RP170691 (ML and AS); funds from the Alkek Center for Molecular Discovery (AS) and Agilent Technologies Center for Excellence in Mass Spectrometry (AS); UH Division of Research, UH College of Natural Sciences and Mathematics, and Department of Biology & Biochemistry, NRUF MINOR CORE 17 Grant to support Seq-N-Edit Core.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Arnold, J.M., Gu, F., Ambati, C.R. et al. UDP-glucose 6-dehydrogenase regulates hyaluronic acid production and promotes breast cancer progression. Oncogene 39, 3089–3101 (2020). https://doi.org/10.1038/s41388-019-0885-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-019-0885-4
This article is cited by
-
Extracellular matrix remodeling in tumor progression and immune escape: from mechanisms to treatments
Molecular Cancer (2023)
-
UDP-glucuronate metabolism controls RIPK1-driven liver damage in nonalcoholic steatohepatitis
Nature Communications (2023)
-
Disruption of sugar nucleotide clearance is a therapeutic vulnerability of cancer cells
Nature (2023)
-
‘Two-faces’ of hyaluronan, a dynamic barometer of disease progression in tumor microenvironment
Discover Oncology (2023)
-
UGDH promotes tumor-initiating cells and a fibroinflammatory tumor microenvironment in ovarian cancer
Journal of Experimental & Clinical Cancer Research (2023)