Abstract
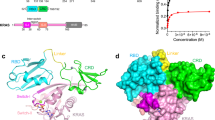
KRAS, HRAS and NRAS proto-oncogenes belong to a family of 40 highly homologous genes, which in turn are a subset of a superfamily of >160 genes encoding small GTPases. RAS proteins consist of a globular G-domain (aa1-166) and a 22-23 aa unstructured hypervariable region (HVR) that mediates membrane targeting. The evolutionary origins of the RAS isoforms, their HVRs and alternative splicing of the KRAS locus has not been explored. We found that KRAS is basal to the RAS proto-oncogene family and its duplication generated HRAS in the common ancestor of vertebrates. In a second round of duplication HRAS generated NRAS and KRAS generated an additional RAS gene we have designated KRASBL, absent in mammals and birds. KRAS4A arose through a duplication and insertion of the 4th exon of NRAS into the 3rd intron of KRAS. We found evolutionary conservation of a short polybasic region (PBR1) in HRAS, NRAS and KRAS4A, a second polybasic region (PBR2) in KRAS4A, two neutralized basic residues (NB) and a serine in KRAS4B and KRASBL, and a modification of the CaaX motif in vertebrates with farnesyl rather than geranylgeranyl polyisoprene lipids, suggesting that a less hydrophobic membrane anchor is critical to RAS protein function. The persistence of four RAS isoforms through >400 million years of evolution argues strongly for differential function.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data analyzed in this report are publicly available from NCBI (https://www.ncbi.nlm.nih.gov), Ensembl (http://www.ensembl.org/) and SIMRBASE (https://genomes.stowers.org). The data utilized are provided in Suppl. File 1.
References
Cox AD, Der CJ. Ras history: the saga continues. Small Gtpases. 2011;1:2–27.
Fernandez-Medarde A, De Las Rivas J, Santos E. 40 years of RAS-a historic overview. Genes (Basel). 2021;12:681.
Prior IA, Lewis PD, Mattos C. A comprehensive survey of Ras mutations in cancer. Cancer Res. 2012;72:2457–67.
Ahearn I, Zhou M, Philips MR. Posttranslational modifications of RAS proteins. Cold Spring Harb Perspect Med. 2018;8:a031484.
Wright LP, Philips MR. Thematic review series: lipid posttranslational modifications. CAAX modification and membrane targeting of Ras. J Lipid Res. 2006;47:883–91.
Gasper R, Wittinghofer F. The Ras switch in structural and historical perspective. Biol Chem. 2019;401:143–63.
Nair A, Kubatzky KF, Saha B. Ras isoforms from lab benches to lives-what are we missing and how far are we? Int J Mol Sci. 2021;22:6508.
Nakamura K, Ichise H, Nakao K, Hatta T, Otani H, Sakagami H, et al. Partial functional overlap of the three ras genes in mouse embryonic development. Oncogene. 2008;27:2961–8.
Mor A, Philips MR. Compartmentalized Ras/MAPK signaling. Annu Rev Immunol. 2006;24:771–800.
Aran V, Prior IA. Compartmentalized Ras signaling differentially contributes to phenotypic outputs. Cell Signal. 2013;25:1748–53.
Campbell SL, Philips MR. Post-translational modification of RAS proteins. Curr Opin Struct Biol. 2021;71:180–92.
Colicelli J. Human RAS superfamily proteins and related GTPases. Sci STKE. 2004;2004:RE13.
van Dam TJ, Bos JL, Snel B. Evolution of the Ras-like small GTPases and their regulators. Small GTPases. 2011;2:4–16.
Wennerberg K, Rossman KL, Der CJ. The Ras superfamily at a glance. J Cell Sci. 2005;118:843–6.
Rojas AM, Fuentes G, Rausell A, Valencia A. The Ras protein superfamily: evolutionary tree and role of conserved amino acids. J Cell Biol. 2012;196:189–201.
Simanshu DK, Nissley DV, McCormick F. RAS proteins and their regulators in human disease. Cell. 2017;170:17–33.
Nuevo-Tapioles C, Philips MR. The role of KRAS splice variants in cancer biology. Front Cell Dev Biol. 2022;10:1033348.
Tsai FD, Lopes MS, Zhou M, Court H, Ponce O, Fiordalisi JJ, et al. K-Ras4A splice variant is widely expressed in cancer and uses a hybrid membrane-targeting motif. Proc Natl Acad Sci USA 2015;112:779–84.
Amendola CR, Mahaffey JP, Parker SJ, Ahearn IM, Chen WC, Zhou M, et al. KRAS4A directly regulates hexokinase 1. Nature. 2019;576:482–6.
Jing H, Zhang X, Wisner SA, Chen X, Spiegelman NA, Linder ME, et al. SIRT2 and lysine fatty acylation regulate the transforming activity of K-Ras4a. Elife. 2017;6:e32436.
Castel P, Dharmaiah S, Sale MJ, Messing S, Rizzuto G, Cuevas-Navarro A, et al. RAS interaction with Sin1 is dispensable for mTORC2 assembly and activity. Proc Natl Acad Sci USA 2021;118:e2103261118.
Lopez JM, Lozano D, Morona R, Gonzalez A. Organization of the catecholaminergic systems in two basal actinopterygian fishes, polypterus senegalus and erpetoichthys calabaricus (actinopterygii: cladistia). J Comp Neurol. 2019;527:437–61.
Venkatesh B, Lee AP, Ravi V, Maurya AK, Lian MM, Swann JB, et al. Elephant shark genome provides unique insights into gnathostome evolution. Nature. 2014;505:174–9.
Miyashita T, Gess RW, Tietjen K, Coates MI. Non-ammocoete larvae of Palaeozoic stem lampreys. Nature. 2021;591:408–12.
Miyashita T, Coates MI, Farrar R, Larson P, Manning PL, Wogelius RA, et al. Hagfish from the Cretaceous Tethys Sea and a reconciliation of the morphological-molecular conflict in early vertebrate phylogeny. Proc Natl Acad Sci USA 2019;116:2146–51.
Wolfe KH, Shields DC. Molecular evidence for an ancient duplication of the entire yeast genome. Nature. 1997;387:708–13.
Garcia-Ranea JA, Valencia A. Distribution and functional diversification of the ras superfamily in Saccharomyces cerevisiae. FEBS Lett. 1998;434:219–25.
Hancock JF, Paterson H, Marshall CJ. A polybasic domain or palmitoylation is required in addition to the CAAX motif to localize p21ras to the plasma membrane. Cell. 1990;63:133–9.
Bivona TG, Quatela SE, Bodemann BO, Ahearn IO, Soskis MJ, Mor A, et al. PKC regulates a farnesyl-electrostatic switch on K-Ras that promotes its association with Bcl-XL on mitochondria and induces apoptosis. Mol Cell. 2006;21:481–93.
Sung PJ, Tsai FD, Vais H, Court H, Yang J, Fehrenbacher N, et al. Phosphorylated K-Ras limits cell survival by blocking Bcl-xL sensitization of inositol trisphosphate receptors. Proc Natl Acad Sci USA 2013;110:20593–8.
Cho KJ, Casteel DE, Prakash P, Tan L, van der Hoeven D, Salim AA, et al. AMPK and endothelial nitric oxide synthase signaling regulates K-ras plasma membrane interactions via cyclic GMP-dependent protein kinase 2. Mol Cell Biol. 2016;36:3086–99.
Wang MT, Holderfield M, Galeas J, Delrosario R, To MD, Balmain A, et al. K-Ras promotes tumorigenicity through suppression of non-canonical wnt signaling. Cell. 2015;163:1237–51.
Senoo H, Murata D, Wai M, Arai K, Iwata W, Sesaki H, et al. KARATE: PKA-induced KRAS4B-RHOA-mTORC2 supercomplex phosphorylates AKT in insulin signaling and glucose homeostasis. Mol Cell. 2021;81:4622–34.e4628.
Reid TS, Terry KL, Casey PJ, Beese LS. Crystallographic analysis of CaaX prenyltransferases complexed with substrates defines rules of protein substrate selectivity. J Mol Biol. 2004;343:417–33.
Silvius JR, l’Heureux F. Fluorimetric evaluation of the affinities of isoprenylated peptides for lipid bilayers. Biochemistry. 1994;33:3014–22.
Simakov O, Marletaz F, Yue JX, O’Connell B, Jenkins J, Brandt A, et al. Deeply conserved synteny resolves early events in vertebrate evolution. Nat Ecol Evol. 2020;4:820–30.
Dehal P, Boore JL. Two rounds of whole genome duplication in the ancestral vertebrate. PLoS Biol. 2005;3:e314.
Ohno S. Gene duplication and the uniqueness of vertebrate genomes circa 1970-99. Semin Cell Dev Biol. 1999;10:517–22.
Lam SD, Babu MM, Lees J, Orengo CA. Biological impact of mutually exclusive exon switching. PLoS Comput Biol. 2021;17:e1008708.
Kolkman JA, Stemmer WP. Directed evolution of proteins by exon shuffling. Nat Biotechnol. 2001;19:423–8.
Patthy L. Genome evolution and the evolution of exon-shuffling-a review. Gene. 1999;238:103–14.
Suyama M. Mechanistic insights into mutually exclusive splicing in dynamin 1. Bioinformatics. 2013;29:2084–7.
Letunic I, Copley RR, Bork P. Common exon duplication in animals and its role in alternative splicing. Hum Mol Genet. 2002;11:1561–7.
Kondrashov FA, Koonin EV. Origin of alternative splicing by tandem exon duplication. Hum Mol Genet. 2001;10:2661–9.
Abascal F, Ezkurdia I, Rodriguez-Rivas J, Rodriguez JM, del Pozo A, Vazquez J, et al. Alternatively spliced homologous exons have ancient origins and are highly expressed at the protein level. PLoS Comput Biol. 2015;11:e1004325.
Wirth A, Labus J, Abdel Galil D, Schill Y, Schmidt S, Bunke T, et al. Palmitoylation of the small GTPase Cdc42 by DHHC5 modulates spine formation and gene transcription. J Biol Chem. 2022;298:102048.
Yap K, Xiao Y, Friedman BA, Je HS, Makeyev EV. Polarizing the neuron through sustained co-expression of alternatively spliced isoforms. Cell Rep. 2016;15:1316–28.
Cohen JB, Broz SD, Levinson AD. Expression of the H-ras proto-oncogene is controlled by alternative splicing. Cell. 1989;58:461–72.
Burd CE, Liu W, Huynh MV, Waqas MA, Gillahan JE, Clark KS, et al. Mutation-specific RAS oncogenicity explains NRAS codon 61 selection in melanoma. Cancer Disco. 2014;4:1418–29.
Cook JH, Melloni GEM, Gulhan DC, Park PJ, Haigis KM. The origins and genetic interactions of KRAS mutations are allele- and tissue-specific. Nat Commun. 2021;12:1808.
Johnson L, Greenbaum D, Cichowski K, Mercer K, Murphy E, Schmitt E, et al. K-ras is an essential gene in the mouse with partial functional overlap with N-ras. Genes Dev. 1997;11:2468–81.
Garcia-Espana A, Chung PJ, Zhao X, Lee A, Pellicer A, Yu J, et al. Origin of the tetraspanin uroplakins and their co-evolution with associated proteins: implications for uroplakin structure and function. Mol Phylogenet Evol. 2006;41:355–67.
Chicote JU, DeSalle R, Garcia-Espana A. Phosphotyrosine phosphatase R3 receptors: origin, evolution and structural diversification. PLoS One. 2017;12:e0172887.
Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–402.
Katoh K, Rozewicki J, Yamada KD. MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Brief Bioinform. 2019;20:1160–6.
Letunic I, Khedkar S, Bork P. SMART: recent updates, new developments and status in 2020. Nucleic Acids Res. 2021;49:D458–D460.
Garcia-Espana A, Mares R, Sun TT, Desalle R. Intron evolution: testing hypotheses of intron evolution using the phylogenomics of tetraspanins. PLoS One. 2009;4:e4680.
UniProt C. UniProt: the universal protein knowledgebase in 2021. Nucleic Acids Res. 2021;49:D480–D489.
Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–7.
Castresana J. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol Biol Evol. 2000;17:540–52.
Gascuel O. BIONJ: an improved version of the NJ algorithm based on a simple model of sequence data. Mol Biol Evol. 1997;14:685–95.
Jones DT, Taylor WR, Thornton JM. The rapid generation of mutation data matrices from protein sequences. Comput Appl Biosci. 1992;8:275–82.
Felsenstein. PHYLIP - phylogeny inference package (Version 3.2). Cladistics. 1989;5:164–6.
Letunic I, Bork P. Interactive Tree Of Life (iTOL) v4: recent updates and new developments. Nucleic Acids Res. 2019;47:W256–W259.
Dereeper A, Guignon V, Blanc G, Audic S, Buffet S, Chevenet F, et al. Phylogeny.fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Res. 2008;36:W465–469.
Hedges SB, Dudley J, Kumar S. TimeTree: a public knowledge-base of divergence times among organisms. Bioinformatics. 2006;22:2971–2.
Talavera D, Hospital A, Orozco M, de la Cruz X. A procedure for identifying homologous alternative splicing events. BMC Bioinforma. 2007;8:260.
Acknowledgements
We thank Sebastien Santini (CNRS/AMU IGS UMR7256) and the PACA Bioinfo platform for the availability and management of the phylogeny.fr website.
Funding
Funding was provided to M.R.P. (NIH R35CA253178).
Author information
Authors and Affiliations
Contributions
AGE mined genomic databases and performed alignment and phylogenetic analysis which was put into the context of RAS membrane targeting by MRP and AGE wrote the manuscript together.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
García-España, A., Philips, M.R. Origin and Evolution of RAS Membrane Targeting. Oncogene 42, 1741–1750 (2023). https://doi.org/10.1038/s41388-023-02672-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-023-02672-z