Abstract
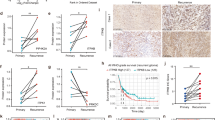
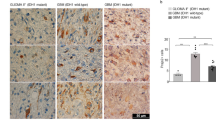
Glioblastoma (GBM) is the most common malignant glioma, with a high recurrence rate and a poor prognosis. However, the molecular mechanism behind the malignant progression of GBM is still unclear. In the present study, through the tandem mass tag (TMT)-based quantitative proteomic analysis of clinical primary and recurrent glioma samples, we identified that aberrant E3 ligase MAEA was expressed in recurrent samples. The results of bioinformatics analysis showed that the high expression of MAEA was related to the recurrence and poor prognosis of glioma and GBM. Functional studies showed that MAEA could promote proliferation, invasion, stemness and temozolomide (TMZ) resistance. Mechanistically, the data indicated that MAEA targeted prolyl hydroxylase domain 3 (PHD3) K159 to promote its K48-linked polyubiquitination and degradation, thus enhancing the stability of HIF-1α, thereby promoting the stemness and TMZ resistance of GBM cells through upregulating CD133. The in vivo experiments further confirmed that knocking down MAEA could inhibit the growth of GBM xenograft tumors. In summary, MAEA enhances the expression of HIF-1α/CD133 through the degradation of PHD3 and promotes the malignant progression of GBM.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
Source data and reagents are available from the corresponding author upon reasonable request.
References
Omuro A, DeAngelis LM. Glioblastoma and other malignant gliomas: a clinical review. JAMA. 2013;310:1842–50.
Zhang AS, Ostrom QT, Kruchko C, Rogers L, Peereboom DM, Barnholtz-Sloan JS. Complete prevalence of malignant primary brain tumors registry data in the United States compared with other common cancers, 2010. Neuro Oncol. 2017;19:726–35.
Hombach-Klonisch S, Mehrpour M, Shojaei S, Harlos C, Pitz M, Hamai A, et al. Glioblastoma and chemoresistance to alkylating agents: Involvement of apoptosis, autophagy, and unfolded protein response. Pharmacol Ther. 2018;184:13–41.
Hervey-Jumper SL, Berger MS. Reoperation for recurrent high-grade glioma: a current perspective of the literature. Neurosurgery. 2014;75:491–9. discussion 8-9
Sharma M, Schroeder JL, Elson P, Meola A, Barnett GH, Vogelbaum MA, et al. Outcomes and prognostic stratification of patients with recurrent glioblastoma treated with salvage stereotactic radiosurgery. J Neurosurg. 2018;131:489–99.
Qin S, Mao Y, Wang H, Duan Y, Zhao L. The interplay between m6A modification and non-coding RNA in cancer stemness modulation: mechanisms, signaling pathways, and clinical implications. Int J Biol Sci. 2021;17:2718–36.
Jia B, Liu W, Gu J, Wang J, Lv W, Zhang W, et al. MiR-7-5p suppresses stemness and enhances temozolomide sensitivity of drug-resistant glioblastoma cells by targeting Yin Yang 1. Exp Cell Res. 2019;375:73–81.
Ulasov IV, Nandi S, Dey M, Sonabend AM, Lesniak MS. Inhibition of Sonic hedgehog and Notch pathways enhances sensitivity of CD133(+) glioma stem cells to temozolomide therapy. Mol Med. 2011;17:103–12.
Nimbalkar VP, Kruthika BS, Sravya P, Rao S, Sugur HS, Verma BK, et al. Differential gene expression in peritumoral brain zone of glioblastoma: role of SERPINA3 in promoting invasion, stemness and radioresistance of glioma cells and association with poor patient prognosis and recurrence. J Neurooncol. 2021;152:55–65.
Nabavi SF, Atanasov AG, Khan H, Barreca D, Trombetta D, Testai L, et al. Targeting ubiquitin-proteasome pathway by natural, in particular polyphenols, anticancer agents: Lessons learned from clinical trials. Cancer Lett. 2018;434:101–13.
Severe N, Dieudonne FX, Marie PJ. E3 ubiquitin ligase-mediated regulation of bone formation and tumorigenesis. Cell Death Dis. 2013;4:e463.
Wang D, Ma L, Wang B, Liu J, Wei W. E3 ubiquitin ligases in cancer and implications for therapies. Cancer Metastasis Rev. 2017;36:683–702.
Lu C, Ning G, Si P, Zhang C, Liu W, Ge W, et al. E3 ubiquitin ligase HECW1 promotes the metastasis of non-small cell lung cancer cells through mediating the ubiquitination of Smad4. Biochem Cell Biol. 2021;99:675–81.
Ji J, Ding K, Luo T, Zhang X, Chen A, Zhang D, et al. TRIM22 activates NF-kappaB signaling in glioblastoma by accelerating the degradation of IkappaBalpha. Cell Death Differ. 2021;28:367–81.
Liu CM, Yu CC, Lin T, Liao YW, Hsieh PL, Yu CH, et al. E3 ligase STUB1 attenuates stemness and tumorigenicity of oral carcinoma cells via transglutaminase 2 regulation. J Formos Med Assoc. 2020;119:1532–8.
Wang Z, Kang L, Zhang H, Huang Y, Fang L, Li M, et al. AKT drives SOX2 overexpression and cancer cell stemness in esophageal cancer by protecting SOX2 from UBR5-mediated degradation. Oncogene. 2019;38:5250–64.
Zhao X, Zhou M, Yang Y, Luo M. The ubiquitin hydrolase OTUB1 promotes glioma cell stemness via suppressing ferroptosis through stabilizing SLC7A11 protein. Bioengineered. 2021;12:12636–45.
Braun B, Pfirrmann T, Menssen R, Hofmann K, Scheel H, Wolf DH. Gid9, a second RING finger protein contributes to the ubiquitin ligase activity of the Gid complex required for catabolite degradation. FEBS Lett. 2011;585:3856–61.
Lampert F, Stafa D, Goga A, Soste MV, Gilberto S, Olieric N, et al. The multi-subunit GID/CTLH E3 ubiquitin ligase promotes cell proliferation and targets the transcription factor Hbp1 for degradation. Elife. 2018;7:e35528.
Wei Q, Pinho S, Dong S, Pierce H, Li H, Nakahara F, et al. MAEA is an E3 ubiquitin ligase promoting autophagy and maintenance of haematopoietic stem cells. Nat Commun. 2021;12:2522.
Rittmann MC, Hussung S, Braun LM, Klar RFU, Biesel EA, Fichtner-Feigl S, et al. Plasma biomarkers for prediction of early tumor recurrence after resection of pancreatic ductal adenocarcinoma. Sci Rep. 2021;11:7499.
Sledzinska P, Bebyn MG, Furtak J, Kowalewski J, Lewandowska MA. Prognostic and Predictive Biomarkers in Gliomas. Int J Mol Sci. 2021;22:10373.
Chen YJ, Wu H, Shen XZ. The ubiquitin-proteasome system and its potential application in hepatocellular carcinoma therapy. Cancer Lett. 2016;379:245–52.
Nakayama K, Frew IJ, Hagensen M, Skals M, Habelhah H, Bhoumik A, et al. Siah2 regulates stability of prolyl-hydroxylases, controls HIF1alpha abundance, and modulates physiological responses to hypoxia. Cell. 2004;117:941–52.
Sun C, Song H, Zhang H, Hou C, Zhai T, Huang L, et al. CD133 expression in renal cell carcinoma (RCC) is correlated with nuclear hypoxia-inducing factor 1alpha (HIF-1alpha). J Cancer Res Clin Oncol. 2012;138:1619–24.
Lai FB, Liu WT, Jing YY, Yu GF, Han ZP, Yang X, et al. Lipopolysaccharide supports maintaining the stemness of CD133(+) hepatoma cells through activation of the NF-kappaB/HIF-1alpha pathway. Cancer Lett. 2016;378:131–41.
Wu Q, Berglund AE, MacAulay RJ, Etame AB. A novel role of BIRC3 in stemness reprogramming of glioblastoma. Int J Mol Sci. 2021;23:297.
Nakod PS, Kim Y, Rao SS. The impact of astrocytes and endothelial cells on glioblastoma stemness marker expression in multicellular spheroids. Cell Mol Bioeng. 2021;14:639–51.
Lee Y, Kim KH, Kim DG, Cho HJ, Kim Y, Rheey J, et al. FoxM1 promotes stemness and radio-resistance of glioblastoma by regulating the master stem cell regulator Sox2. PLoS One. 2015;10:e0137703.
Zhang C, Mukherjee S, Tucker-Burden C, Ross JL, Chau MJ, Kong J, et al. TRIM8 regulates stemness in glioblastoma through PIAS3-STAT3. Mol Oncol. 2017;11:280–94.
Dopeso H, Jiao HK, Cuesta AM, Henze AT, Jurida L, Kracht M, et al. PHD3 controls lung cancer metastasis and resistance to EGFR inhibitors through TGFalpha. Cancer Res. 2018;78:1805–19.
Henze AT, Garvalov BK, Seidel S, Cuesta AM, Ritter M, Filatova A, et al. Loss of PHD3 allows tumours to overcome hypoxic growth inhibition and sustain proliferation through EGFR. Nat Commun. 2014;5:5582.
Shi M, Dai WQ, Jia RR, Zhang QH, Wei J, Wang YG, et al. APC(CDC20)-mediated degradation of PHD3 stabilizes HIF-1a and promotes tumorigenesis in hepatocellular carcinoma. Cancer Lett. 2021;496:144–55.
Sen Banerjee S, Thirunavukkarasu M, Tipu Rishi M, Sanchez JA, Maulik N, Maulik G. HIF-prolyl hydroxylases and cardiovascular diseases. Toxicol Mech Methods. 2012;22:347–58.
Chan DA, Sutphin PD, Yen SE, Giaccia AJ. Coordinate regulation of the oxygen-dependent degradation domains of hypoxia-inducible factor 1 alpha. Mol Cell Biol. 2005;25:6415–26.
Xia X, Wang S, Ni B, Xing S, Cao H, Zhang Z, et al. Hypoxic gastric cancer-derived exosomes promote progression and metastasis via MiR-301a-3p/PHD3/HIF-1alpha positive feedback loop. Oncogene. 2020;39:6231–44.
Tanaka T, Li TS, Urata Y, Goto S, Ono Y, Kawakatsu M, et al. Increased expression of PHD3 represses the HIF-1 signaling pathway and contributes to poor neovascularization in pancreatic ductal adenocarcinoma. J Gastroenterol. 2015;50:975–83.
Ohnishi S, Maehara O, Nakagawa K, Kameya A, Otaki K, Fujita H, et al. Hypoxia-inducible factors activate CD133 promoter through ETS family transcription factors. PLoS One. 2013;8:e66255.
Xu H, Jin J, Chen Y, Wu G, Zhu H, Wang Q, et al. GBP3 promotes glioblastoma resistance to temozolomide by enhancing DNA damage repair. Oncogene. 2022;41:3876–85.
Wu X, Zhou Z, Xu S, Liao C, Chen X, Li B, et al. Extracellular vesicle packaged LMP1-activated fibroblasts promote tumor progression via autophagy and stroma-tumor metabolism coupling. Cancer Lett. 2020;478:93–106.
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (No. 81974466), the Natural Science Foundation of Changsha, China (kq2202124), the Hunan Provincial Innovation Foundation for Postgraduate (No. CX20200382) and the Fundamental Research Funds for the Central Universities of Central South University (No. 1053320192726, 1053320212989).
Author information
Authors and Affiliations
Contributions
PJZ and XZP designed the experiments, performed research and analyzed the data. SYT and LFS collected the tissue samples for proteomics analysis and analyzed data. KZ participated in xenograft experiments, processed tissue samples and analyzed data. ZKT generated constructs. JWP and LFY directed the research and analyzed the data. PJZ, DL and LFY wrote the manuscript. All authors approved final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics statement
The experimental protocol was approved by the Ethics Committee of Xiangya Hospital of Central South University. Animals were raised and operated in accordance with the guidelines formulated by the Medical Research Animal Ethics Committee of Central South University.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhou, P., Peng, X., Tang, S. et al. E3 ligase MAEA-mediated ubiquitination and degradation of PHD3 promotes glioblastoma progression. Oncogene 42, 1308–1320 (2023). https://doi.org/10.1038/s41388-023-02644-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-023-02644-3