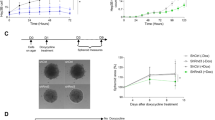
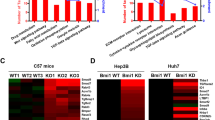
Abstract
Hepatocellular carcinoma (HCC) is one of the deadliest cancers. The retinoblastoma protein (RB1), a regulator of cell proliferation, is functionally inactivated in HCC by CYCLIN D/E-mediated phosphorylation. However, the mechanism of RB1-inactivation is unclear because only small percentages of HCCs exhibit amplification of CYCLIN D/E or mutations in the CDK-inhibitory genes. We show that FOXM1, which is overexpressed and critical for HCC, plays essential roles in inactivating RB1 and suppressing RB1-induced senescence of the HCC cells. Mechanistically, FOXM1 binds RB1 and DNMT3B to repress the expression of FOXO1, leading to a decrease in the levels of the CDK-inhibitors, creating an environment for phosphorylation and inactivation of RB1. Consistent with that, inhibition of FOXM1 causes increased expression of FOXO1 with consequent activation of RB1, leading to senescence of the HCC cells, in vitro and in vivo. Also, repression-deficient mutants of FOXM1 induce senescence that is blocked by depletion of RB1 or FOXO1. We provide evidence that human HCCs rely upon this FOXM1–FOXO1 axis for phosphorylation and inactivation of RB1. The observations demonstrate the existence of a new autoregulatory loop of RB1-inactivation in HCC involving a FOXM1–FOXO1 axis that is required for phosphorylation of RB1 and for aggressive progression of HCC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Change history
07 September 2022
A Correction to this paper has been published: https://doi.org/10.1038/s41388-022-02436-1
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29.
Akinyemiju T, Abera S, Ahmed M, Alam N, Alemayohu MA, Allen C, et al. The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level results from the global burden of disease study 2015. JAMA Oncol. 2017;3:1683–91.
Park JW, Kim YJ, Kim DY, Bae SH, Paik SW, Lee YJ, et al. Sorafenib with or without concurrent transarterial chemoembolization in patients with advanced hepatocellular carcinoma: the phase III STAH trial. J Hepatol. 2019;70:684–91.
Zucman-Rossi J, Villanueva A, Nault JC, Llovet JM. Genetic landscape and biomarkers of hepatocellular carcinoma. Gastroenterology. 2015;149:1226.
Midorikawa Y, Yamamoto S, Tatsuno K, Renard-Guillet C, Tsuji S, Hayashi A, et al. Accumulation of molecular aberrations distinctive to hepatocellular carcinoma progression. Cancer Res. 2020;80:3810–9.
Ahn SM, Jang SJ, Shim JH, Kim D, Hong SM, Sung CO, et al. Genomic portrait of resectable hepatocellular carcinomas: implications of RB1 and FGF19 aberrations for patient stratification. Hepatology. 2014;60:1972–82.
Reed CA, Mayhew CN, McClendon AK, Yang X, Witkiewicz A, Knudsen ES. RB has a critical role in mediating the in vivo checkpoint response, mitigating secondary DNA damage and suppressing liver tumorigenesis initiated by aflatoxin B1. Oncogene. 2009;28:4434–43.
Viatour P, Ehmer U, Saddic LA, Dorrell C, Andersen JB, Lin CW, et al. Notch signaling inhibits hepatocellular carcinoma following inactivation of the RB pathway. J Exp Med. 2011;208:1963–76.
Rubin SM, Sage J, Skotheim JM. Integrating old and new paradigms of G1/S control. Mol Cell. 2020;80:183–92.
Baker GL, Landis MW, Hinds PW. Multiple functions of D-type cyclins can antagonize pRb-mediated suppression of proliferation. Cell Cycle. 2005;4:330–8.
Guichard C, Amaddeo G, Imbeaud S, Ladeiro Y, Pelletier L, Ben Maad I, et al. Integrated analysis of somatic mutations and focal copy-number changes identifies key genes and pathways in hepatocellular carcinoma. Nat Genet. 2012;44:694–U120.
Barsotti AM, Prives C. Pro-proliferative FoxM1 is a target of p53-mediated repression. Oncogene. 2009;28:4295–305.
Kopanja D, Pandey A, Kiefer M, Wang Z, Chandan N, Carr JR, et al. Essential roles of FoxM1 in Ras-induced liver cancer progression and in cancer cells with stem cell features. J Hepatol. 2015;63:429–36.
Song BN, Chu IS. A gene expression signature of FOXM1 predicts the prognosis of hepatocellular carcinoma. Exp Mol Med. 2018;50:e418.
Kalinichenko VV, Major ML, Wang X, Petrovic V, Kuechle J, Yoder HM, et al. Foxm1b transcription factor is essential for development of hepatocellular carcinomas and is negatively regulated by the p19ARF tumor suppressor. Genes Dev. 2004;18:830–50.
Carr JR, Kiefer MM, Park HJ, Li J, Wang Z, Fontanarosa J, et al. FoxM1 regulates mammary luminal cell fate. Cell Rep. 2012;1:715–29.
Chand V, Pandey A, Kopanja D, Guzman G, Raychaudhuri P. Opposing roles of the Fork-head box genes FoxM1 and FoxA2 in liver cancer. Mol Cancer Res. 2019;17:1063–74.
Major ML, Lepe R, Costa RH. Forkhead box M1B transcriptional activity requires binding of Cdk-cyclin complexes for phosphorylation-dependent recruitment of p300/CBP coactivators. Mol Cell Biol. 2004;24:2649–61. (Research Support, U.S. Govt, P.H.S.)
Mukhopadhyay NK, Chand V, Pandey A, Kopanja D, Carr JR, Chen YJ, et al. Plk1 regulates the repressor function of FoxM1b by inhibiting its interaction with the retinoblastoma protein. Sci Rep. 2017;7:46017.
Fu Z, Malureanu L, Huang J, Wang W, Li H, van Deursen JM, et al. Plk1-dependent phosphorylation of FoxM1 regulates a transcriptional programme required for mitotic progression. Nat Cell Biol. 2008;10:1076–82.
Chen YJ, Dominguez-Brauer C, Wang Z, Asara JM, Costa RH, Tyner AL, et al. A conserved phosphorylation site within the forkhead domain of FoxM1B is required for its activation by cyclin-CDK1. J Biol Chem. 2009;284:30695–707.
Jiao W, Datta J, Lin HM, Dundr M, Rane SG. Nucleocytoplasmic shuttling of the retinoblastoma tumor suppressor protein via Cdk phosphorylation-dependent nuclear export. J Biol Chem. 2006;281:38098–108.
Laoukili J, Kooistra MR, Bras A, Kauw J, Kerkhoven RM, Morrison A, et al. FoxM1 is required for execution of the mitotic programme and chromosome stability. Nat Cell Biol. 2005;7:126–36.
Wang IC, Chen YJ, Hughes D, Petrovic V, Major ML, Park HJ, et al. Forkhead box M1 regulates the transcriptional network of genes essential for mitotic progression and genes encoding the SCF (Skp2-Cks1) ubiquitin ligase. Mol Cell Biol. 2005;25:10875–94.
Park HJ, Wang Z, Costa RH, Tyner A, Lau LF, Raychaudhuri P. An N-terminal inhibitory domain modulates activity of FoxM1 during cell cycle. Oncogene. 2008;27:1696–704.
Marceau AH, Brison CM, Nerli S, Arsenault HE, McShan AC, Chen EF, et al. An order-to-disorder structural switch activates the FoxM1 transcription factor. Elife. 2019;8:e46131.
Diep CH, Charles NJ, Gilks CB, Kalloger SE, Argenta PA, Lange CA. Progesterone receptors induce FOXO1-dependent senescence in ovarian cancer cells. Cell Cycle. 2013;12:1433–49.
Shang YK, Li F, Zhang Y, Liu ZK, Wang ZL, Bian HJ, et al. Systems analysis of key genes and pathways in the progression of hepatocellular carcinoma. Medicine. 2018;97:e10892.
Liu JK, Liu ZY, Li W, Zhang SR. SOCS2 is a potential prognostic marker that suppresses the viability of hepatocellular carcinoma cells. Oncol Lett. 2021;21:399.
Nowak K, Killmer K, Gessner C, Lutz W. E2F-1 regulates expression of FOXO1 and FOXO3a. Biochim Biophys Acta. 2007;1769:244–52.
Park HJ, Gusarova G, Wang Z, Carr JR, Li J, Kim KH, et al. Deregulation of FoxM1b leads to tumour metastasis. EMBO Mol Med. 2011;3:21–34.
Ruscetti M, Morris JP, Mezzadra R, Russell J, Leibold J, Romesser PB, et al. Senescence-induced vascular remodeling creates therapeutic vulnerabilities in pancreas cancer. Cell. 2020;181:424.
Chicas A, Wang XW, Zhang CL, McCurrach M, Zhao Z, Mert O, et al. Dissecting the unique role of the retinoblastoma tumor suppressor during cellular senescence. Cancer Cell. 2010;17:376–87.
Dimri GP, Acosta M, Campisi J. Regulation of E2F related genes during cellular senescence. Mol Biol Cell. 1996;7:3102–3102.
Dimri GP, Testori A, Acosta M, Campisi J. Replicative senescence, aging and growth-regulatory transcription factors. Biol Signal. 1996;5:154–62.
Nevins JR. The Rb/E2F pathway and cancer. Hum Mol Genet. 2001;10:699–703. (Review)
Narasimha AM, Kaulich M, Shapiro GS, Choi YJ, Sicinski P, Dowdy SF. Cyclin D activates the Rb tumor suppressor by mono-phosphorylation. Elife. 2014;3:e02872.
Topacio BR, Zatulovskiy E, Cristea S, Xie S, Tambo CS, Rubin SM, et al. Cyclin D-Cdk4,6 drives cell-cycle progression via the retinoblastoma protein’s C-terminal helix. Mol Cell. 2019;74:758–70.e754.
Knudsen ES, Pruitt SC, Hershberger PA, Witkiewicz AK, Goodrich DW. Cell cycle and beyond: exploiting new RB1 controlled mechanisms for cancer therapy. Trends Cancer. 2019;5:308–24.
Manning BD, Toker A. AKT/PKB signaling: navigating the network. Cell. 2017;169:381–405.
Benevolenskaya EV, Murray HL, Branton P, Young RA, Kaelin WG Jr. Binding of pRB to the PHD protein RBP2 promotes cellular differentiation. Mol Cell. 2005;18:623–35.
Berman SD, Yuan TL, Miller ES, Lee EY, Caron A, Lees JA. The retinoblastoma protein tumor suppressor is important for appropriate osteoblast differentiation and bone development. Mol Cancer Res. 2008;6:1440–51.
Calo E, Quintero-Estades JA, Danielian PS, Nedelcu S, Berman SD, Lees JA. Rb regulates fate choice and lineage commitment in vivo. Nature. 2010;466:1110–4.
Zappia MP, Rogers A, Islam A, Frolov MV. Rbf activates the myogenic transcriptional program to promote skeletal muscle differentiation. Cell Rep. 2019;26:702–19.e706.
Coppe JP, Desprez PY, Krtolica A, Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol. 2010;5:99–118.
Pozo OJ, Barreda M, Sancho JV, Hernandez F, Ll Lliberia J, Cortes MA, et al. Multiresidue pesticide analysis of fruits by ultra-performance liquid chromatography tandem mass spectrometry. Anal Bioanal Chem. 2007;389:1765–71.
Acknowledgements
This work was supported by a grant (I01 BX000131) from the Department of Veterans Affair (Biomedical Laboratory Research Development Service), and a grant from the NIH (5 RO1 CA243247) to PR. The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government. EB acknowledges support from NIH grant (RO1 CA211095).
Author information
Authors and Affiliations
Contributions
VC and PR designed research, analyzed data, and wrote the paper. VC performed the experiments. XL, GG, and EB contributed critical reagents.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Chand, V., Liao, X., Guzman, G. et al. Hepatocellular carcinoma evades RB1-induced senescence by activating the FOXM1–FOXO1 axis. Oncogene 41, 3778–3790 (2022). https://doi.org/10.1038/s41388-022-02394-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-022-02394-8
This article is cited by
-
M7G methylated core genes (METTL1 and WDR4) and associated RNA risk signatures are associated with prognosis and immune escape in HCC
BMC Medical Genomics (2023)
-
FOXM1 promotes neurofibromatosis type 1-associated malignant peripheral nerve sheath tumor progression in a NUF2-dependent manner
Cancer Gene Therapy (2023)
-
DNMT3B overexpression downregulates genes with CpG islands, common motifs, and transcription factor binding sites that interact with DNMT3B
Scientific Reports (2022)