Abstract
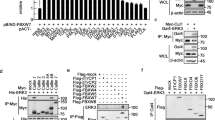
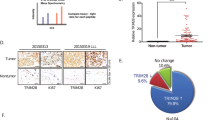
CBL family proteins (CBL, CBLB and CBLC in mammals) are E3 ubiquitin ligases of protein tyrosine kinases. CBL mediates the lysosomal degradation of activated EGFR through K63-linked ubiquitination, while CBLC has an oncogenic function by positively regulating EGFR activation through K6 and K11-linked ubiquitination in EGFR mutant lung adenocarcinoma (LAD). Here, we used immunoprecipitation and mass spectrometry to study the CBLC interactome, and found that CBLC is also involved in cell cycle regulation by stabilizing Aurora kinase A (AURKA). CBLC interacted with the kinase domain of AURKA and positively regulated the stability of AURKA by conjugating monoubiquitination and K11/K63-linked polyubiquitination, which are protective from degrading K11/K48 polyubiquitination. CBLC depletion markedly decreased the half-life of AURKA in cycloheximide-treated LAD cells. When LAD cells were synchronized with double thymidine block at the G1/S boundary and then released into mitotic arrest, CBLC depletion delayed the accumulation and activation of AURKA and prevented cancer cells from entering mitosis. CBLC deficiency significantly delayed cell cycle progression, reduced the mitotic population, and increased apoptosis of LAD cells. Targeting CBLC inhibited tumor growth of LAD cells and enhanced their sensitivity to paclitaxel in xenograft models. Immunohistochemical staining of the tissue microarray also revealed a positive correlation between the expression of CBLC and AURKA in normal and LAD tissues, further supporting the positive regulation of AURKA expression by CBLC. In summary, these findings indicate that the oncogenic E3 ligase CBLC plays a role in mitotic entry by stabilizing AURKA via ubiquitination in LAD. This work demonstrates that targeting CBLC combined with paclitaxel might be a potential option for the treatment of LAD patients who have no available targeted therapies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Genova C, Rossi G, Tagliamento M, Rijavec E, Biello F, Cerbone L, et al. Targeted therapy of oncogenic-driven advanced non-small cell lung cancer: Recent advances and new perspectives. Expert Rev Respir Med 2020;14:367–83.
Thien CB, Langdon WY. Cbl: Many adaptations to regulate protein tyrosine kinases. Nat Rev Mol Cell Biol. 2001;2:294–307.
Keane MM, Ettenberg SA, Nau MM, Banerjee P, Cuello M, Penninger J, et al. cbl-3: A new mammalian cbl family protein. Oncogene. 1999;18:3365–75.
Levkowitz G, Waterman H, Ettenberg SA, Katz M, Tsygankov AY, Alroy I, et al. Ubiquitin ligase activity and tyrosine phosphorylation underlie suppression of growth factor signaling by c-Cbl/Sli-1. Mol Cell. 1999;4:1029–40.
Schmidt MH, Dikic I. The Cbl interactome and its functions. Nat Rev Mol Cell Biol. 2005;6:907–18.
Kim M, Tezuka T, Suziki Y, Sugano S, Hirai M, Yamamoto T. Molecular cloning and characterization of a novel cbl-family gene, cbl-c. Gene. 1999;239:145–54.
Griffiths EK, Sanchez O, Mill P, Krawczyk C, Hojilla CV, Rubin E, et al. Cbl-3-deficient mice exhibit normal epithelial development. Mol Cell Biol. 2003;23:7708–18.
Kim B, Lee HJ, Choi HY, Shin Y, Nam S, Seo G, et al. Clinical validity of the lung cancer biomarkers identified by bioinformatics analysis of public expression data. Cancer Res. 2007;67:7431–8.
Lee WY, Goh G, Chia J, Boey A, Gunko NV, Bard F. The ubiquitin ligase CBLC maintains the network organization of the golgi apparatus. PLoS One. 2015;10:e0138789.
Frankum J, Moudry P, Brough R, Hodny Z, Ashworth A, Bartek J, et al. Complementary genetic screens identify the E3 ubiquitin ligase CBLC, as a modifier of PARP inhibitor sensitivity. Oncotarget. 2015;6:10746–58.
Yau R, Rape M. The increasing complexity of the ubiquitin code. Nat Cell Biol. 2016;18:579–86.
Senft D, Qi J, Ronai ZA. Ubiquitin ligases in oncogenic transformation and cancer therapy. Nat Rev Cancer. 2018;18:69–88.
Hong SY, Kao YR, Lee TC, Wu CW. Upregulation of E3 ubiquitin ligase CBLC enhances EGFR dysregulation and signaling in lung adenocarcinoma. Cancer Res. 2018;78:4984–96.
Lens SM, Voest EE, Medema RH. Shared and separate functions of polo-like kinases and aurora kinases in cancer. Nat Rev Cancer. 2010;10:825–41.
Lindon C, Grant R, Min M. Ubiquitin-mediated degradation of aurora kinases. Front Oncol. 2015;5:307.
Sadowski M, Suryadinata R, Tan AR, Roesley SN, Sarcevic B. Protein monoubiquitination and polyubiquitination generate structural diversity to control distinct biological processes. IUBMB Life. 2012;64:136–42.
Nikonova AS, Astsaturov I, Serebriiskii IG, Dunbrack RL Jr., Golemis EA. Aurora A kinase (AURKA) in normal and pathological cell division. Cell Mol Life Sci. 2013;70:661–87.
Abdelbaki A, Akman HB, Poteau M, Grant R, Gavet O, Guarguaglini G et al. AURKA destruction is decoupled from its activity at mitotic exit but is essential to suppress interphase activity. J Cell Sci. 2020;133:jcs243071.
Otto T, Horn S, Brockmann M, Eilers U, Schuttrumpf L, Popov N, et al. Stabilization of N-Myc is a critical function of Aurora A in human neuroblastoma. Cancer Cell. 2009;15:67–78.
Bertolin G, Tramier M. Insights into the non-mitotic functions of Aurora kinase A: more than just cell division. Cell Mol Life Sci. 2020;77:1031–47.
Anand S, Penrhyn-Lowe S, Venkitaraman AR. AURORA-A amplification overrides the mitotic spindle assembly checkpoint, inducing resistance to Taxol. Cancer Cell. 2003;3:51–62.
Hannak E, Kirkham M, Hyman AA, Oegema K. Aurora-A kinase is required for centrosome maturation in Caenorhabditis elegans. J Cell Biol. 2001;155:1109–16.
Ke YW, Dou Z, Zhang J, Yao XB. Function and regulation of Aurora/Ipl1p kinase family in cell division. Cell Res. 2003;13:69–81.
Hirota T, Kunitoku N, Sasayama T, Marumoto T, Zhang D, Nitta M, et al. Aurora-A and an interacting activator, the LIM protein Ajuba, are required for mitotic commitment in human cells. Cell. 2003;114:585–98.
Cowley DO, Rivera-Perez JA, Schliekelman M, He YJ, Oliver TG, Lu L, et al. Aurora-A kinase is essential for bipolar spindle formation and early development. Mol Cell Biol. 2009;29:1059–71.
Lin X, Xiang X, Hao L, Wang T, Lai Y, Abudoureyimu M, et al. The role of Aurora-A in human cancers and future therapeutics. Am J Cancer Res. 2020;10:2705–29.
Reiter R, Gais P, Jutting U, Steuer-Vogt MK, Pickhard A, Bink K, et al. Aurora kinase A messenger RNA overexpression is correlated with tumor progression and shortened survival in head and neck squamous cell carcinoma. Clin Cancer Res. 2006;12:5136–41.
Yan M, Wang C, He B, Yang M, Tong M, Long Z, et al. Aurora-A kinase: A potent oncogene and target for cancer therapy. Med Res Rev. 2016;36:1036–79.
Du R, Huang C, Liu K, Li X, Dong Z. Targeting AURKA in cancer: Molecular mechanisms and opportunities for Cancer therapy. Mol Cancer. 2021;20:15.
Willems E, Dedobbeleer M, Digregorio M, Lombard A, Lumapat PN, Rogister B. The functional diversity of Aurora kinases: A comprehensive review. Cell Div. 2018;13:7.
Teixeira LK, Reed SI. Ubiquitin ligases and cell cycle control. Annu Rev Biochem. 2013;82:387–414.
Bassermann F, Eichner R, Pagano M. The ubiquitin proteasome system - implications for cell cycle control and the targeted treatment of cancer. Biochim Biophys Acta. 2014;1843:150–62.
Moghe S, Jiang F, Miura Y, Cerny RL, Tsai MY, Furukawa M. The CUL3-KLHL18 ligase regulates mitotic entry and ubiquitylates Aurora-A. Biol Open. 2012;1:82–91.
Gilberto S, Peter M. Dynamic ubiquitin signaling in cell cycle regulation. J Cell Biol. 2017;216:2259–71.
Otto T, Sicinski P. Cell cycle proteins as promising targets in cancer therapy. Nat Rev Cancer. 2017;17:93–115.
Galetta D, Cortes-Dericks L. Promising therapy in lung cancer: Spotlight on aurora kinases. Cancers (Basel) 2020;12.
Lu LY, Wood JL, Ye L, Minter-Dykhouse K, Saunders TL, Yu X, et al. Aurora A is essential for early embryonic development and tumor suppression. J Biol Chem. 2008;283:31785–90.
Mou PK, Yang EJ, Shi C, Ren G, Tao S, Shim JS. Aurora kinase A, a synthetic lethal target for precision cancer medicine. Exp Mol Med. 2021;53:835–47.
Do TV, Hirst J, Hyter S, Roby KF, Godwin AK. Aurora A kinase regulates non-homologous end-joining and poly(ADP-ribose) polymerase function in ovarian carcinoma cells. Oncotarget. 2017;8:50376–92.
Hirst J, Godwin AK. AURKA inhibition mimics BRCAness. Aging (Albany NY). 2017;9:1945–6.
Caracciolo D, Riillo C, Arbitrio M, Di Martino MT, Tagliaferri P, Tassone P. Error-prone DNA repair pathways as determinants of immunotherapy activity: an emerging scenario for cancer treatment. Int J Cancer. 2020;147:2658–68.
Hsu FF, Chiang MT, Li FA, Yeh CT, Lee WH, Chau LY. Acetylation is essential for nuclear heme oxygenase-1-enhanced tumor growth and invasiveness. Oncogene. 2017;36:6805–14.
Acknowledgements
This work was supported by the Ministry of Science and Technology of Taiwan (MOST 109-2314-B-567-001-MY2 and MOST 110-2314-B-567-004 to SYH, MOST 109-2320-B-A49-001 to CWW) and Cardinal Tien Hospital (CTH 110A-2207 to SYH and CTH 110A-NDMC-2227 to CYW). We also thank the core facilities of IBMS, funded by Academia Sinica Core Facility and Innovative Instrument Projects (AS-CFII-108-115, AS-CFII-108-113).
Author information
Authors and Affiliations
Contributions
SYH, SHH, CYW, and CWW conceived the project. SYH, YCL, YRK, MHL, and YPL designed and performed the experiments. All authors analyzed and interpreted the data. SYH, SHH, CYW, and CWW drafted the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Hong, SY., Lu, YC., Hsiao, SH. et al. Stabilization of AURKA by the E3 ubiquitin ligase CBLC in lung adenocarcinoma. Oncogene 41, 1907–1917 (2022). https://doi.org/10.1038/s41388-022-02180-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-022-02180-6
This article is cited by
-
GABA induced by sleep deprivation promotes the proliferation and migration of colon tumors through miR-223-3p endogenous pathway and exosome pathway
Journal of Experimental & Clinical Cancer Research (2023)
-
E2F1-mediated KDM4A-AS1 up-regulation promotes EMT of hepatocellular carcinoma cells by recruiting ILF3 to stabilize AURKA mRNA
Cancer Gene Therapy (2023)
-
Mannose: a potential saccharide candidate in disease management
Medicinal Chemistry Research (2023)