Abstract
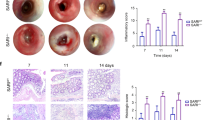
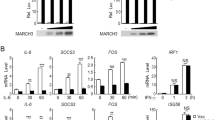
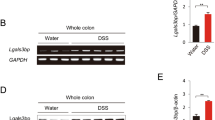
Inflammatory bowel diseases, like ulcerative colitis and Crohn’s disease are frequently accompanied by colorectal cancers. However, the mechanisms underlying colitis-associated cancers are not fully understood. Src Kinase Associated Phosphoprotein 2 (SKAP2), a substrate of Src family kinases, is highly expressed in macrophages. Here, we examined the effects of SKAP2 on inflammatory responses in a mouse model of tumorigenesis with colitis induced by azoxymethane/dextran sulfate sodium. SKAP2 knockout increased the severity of colitis and tumorigenesis, as well as lipopolysaccharide (LPS) induced acute inflammation. SKAP2 attenuated inflammatory signaling in macrophages induced by uptake of cancer cell-derived exosomes. SKAP2−/− mice were characterized by the activation of NF-κB signaling and the upregulation and release of cytokines including TNFα, IL-1β, IL-6, CXCL-9/-10/-13, and sICAM1; SKAP2 overexpression attenuated NF-κB activation. Mechanistically, SKAP2 formed a complex with the SHP-1 tyrosine phosphatase via association with the Sirpα transmembrane receptor. SKAP2 also physically associated with the TIR domain of MyD88, TIRAP, and TRAM, adaptors of toll-like receptor 4 (TLR4). SKAP2-mediated recruitment of the Sirpα/SHP-1 complex to TLR4 attenuated inflammatory responses, whereas direct interaction of SKAP2 with SHP-2 decreased SHP-2 activation. SHP-2 is required for efficient NF-κB activation and suppresses the TRAM/TRIF-INFβ pathway; therefore, SKAP2-mediated SHP-2 inhibition affected two signaling axes from TLR4. The present findings indicate that SKAP2 prevents excess inflammation by inhibiting the TLR4-NF-κB pathway, and it activates the TLR4-IFNβ pathway through SHP-1 and SHP-2, thereby suppressing inflammation-mediated tumorigenesis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Bourette RP, Thérier J, Mouchiroud G. Macrophage colony-stimulating factor receptor induces tyrosine phosphorylation of SKAP55R adaptor and its association with actin. Cell Signal. 2005;17:941–9.
Reinhold A, Reimann S, Reinhold D, Schraven B, Togni M. Expression of SKAP-HOM in DCs is required for an optimal immune response in vivo. J Leukoc Biol. 2009;86:61–71.
Swanson KD, Tang Y, Ceccarelli DF, Poy F, Sliwa JP, Neel BG, et al. The Skap-hom dimerization and PH domains comprise a 3’-phosphoinositide-gated molecular switch. Mol Cell. 2008;32:564–75.
Tanaka M, Shimamura S, Kuriyama S, Maeda D, Goto A, Aiba N. SKAP2 Promotes Podosome Formation to Facilitate Tumor-Associated Macrophage Infiltration and Metastatic Progression. Cancer Res. 2016;76:358–69.
Boras M, Volmering S, Bokemeyer A, Rossaint J, Block H, Bardel B, et al. Skap2 is required for β(2) integrin-mediated neutrophil recruitment and functions. J Exp Med. 2017;214:851–74.
Shaban L, Nguyen GT, Mecsas-Faxon BD, Swanson KD, Tan S, Mecsas J. Yersinia pseudotuberculosis YopH targets SKAP2-dependent and independent signaling pathways to block neutrophil antimicrobial mechanisms during infection. PLoS Pathog. 2020;16:e1008576.
Nguyen GT, Shaban L, Mack M, Swanson KD, Bunnell SC, Sykes DB, et al. SKAP2 is required for defense against K. pneumoniae infection and neutrophil respiratory burst. Elife 2020;9:e56656.
Timms JF, Swanson KD, Marie-Cardine A, Raab M, Rudd CE, Schraven B, et al. SHPS-1 is a scaffold for assembling distinct adhesion-regulated multi-protein complexes in macrophages. Curr Biol. 1999;9:927–30.
Alenghat FJ, Baca QJ, Rubin NT, Pao LI, Matozaki T, Lowell CA, et al. Macrophages require Skap2 and Sirpα for integrin-stimulated cytoskeletal rearrangement. J Cell Sci. 2012;125:5535–45.
Shi L, Han X, Li JX, Liao YT, Kou FS, Wang ZB, et al. Identification of differentially expressed genes in ulcerative colitis and verification in a colitis mouse model by bioinformatics analyses. World J Gastroenterol. 2020;26:5983–96.
Yashiro M. Ulcerative colitis-associated colorectal cancer. World J Gastroenterol. 2014;20:16389–97.
Snider AJ, Bialkowska AB, Ghaleb AM, Yang VW, Obeid LM, Hannun YA. Murine model for colitis-associated cancer of the colon. Methods Mol Biol. 2016;1438:245–54.
Waldner MJ, Neurath MF. Mechanisms of immune signaling in colitis-associated cancer. Cell Mol Gastroenterol Hepatol. 2015;1:6–16.
Fukata M, Hernandez Y, Conduah D, Cohen J, Chen A, Breglio K, et al. Innate immune signaling by Toll-like receptor-4 (TLR4) shapes the inflammatory microenvironment in colitis-associated tumors. Inflamm Bowel Dis. 2009;15:997–1006.
Rossol M, Heine H, Meusch U, Quandt D, Klein C, Sweet MJ, et al. LPS-induced cytokine production in human monocytes and macrophages. Crit Rev Immunol. 2011;31:379–446.
Hoogland IC, Houbolt C, van Westerloo DJ, van Gool WA, van de Beek D. Systemic inflammation and microglial activation: systematic review of animal experiments. J Neuroinflammation. 2015;12:114.
Corrin B. Lung pathology in septic shock. J Clin Pathol. 1980;33:891–4.
Chattopadhyay S, Sen GC. Tyrosine phosphorylation in toll-like receptor signaling. Cytokine Growth Factor Rev. 2014;25:533–41.
Antwi-Baffour SS. Molecular characterisation of plasma membrane-derived vesicles. J Biomed Sci. 2015;22:68.
Ciardiello C, Cavallini L, Spinelli C, Yang J, Reis-Sobreiro M, de Candia P, et al. Focus on extracellular vesicles: new frontiers of cell-to-cell communication in cancer. Int J Mol Sci. 2016;17:175.
Qian Z, Shen Q, Yang X, Qiu Y, Zhang W. The role of extracellular vesicles: an epigenetic view of the cancer microenvironment. Biomed Res Int. 2015;2015:649161.
Steinbichler TB, Dudás J, Riechelmann H, Skvortsova II. The role of exosomes in cancer metastasis. Semin Cancer Biol. 2017;44:170–81.
Paschon V, Takada SH, Ikebara JM, Sousa E, Raeisossadati R, Ulrich H, et al. Interplay between exosomes, microRNAs and toll-like receptors in brain disorders. Mol Neurobiol. 2016;53:2016–28.
Zhang X, Shi H, Yuan X, Jiang P, Qian H, Xu W. Tumor-derived exosomes induce N2 polarization of neutrophils to promote gastric cancer cell migration. Mol Cancer. 2018;17:146.
Barichello T, Generoso JS, Simões LR, Elias SG, Quevedo J. Role of oxidative stress in the pathophysiology of pneumococcal meningitis. Oxid Med Cell Longev. 2013;2013:371465.
Kagan JC, Su T, Horng T, Chow A, Akira S, Medzhitov R. TRAM couples endocytosis of Toll-like receptor 4 to the induction of interferon-beta. Nat Immunol. 2008;9:361–8.
Kuzmich NN, Sivak KV, Chubarev VN, Porozov YB, Savateeva-Lyubimova TN, Peri F. TLR4 Signaling pathway modulators as potential therapeutics in inflammation and sepsis. Vaccines. 2017;5:34.
Li P, Wu YH, Zhu YT, Li MX, Pei HH. Requirement of Rab21 in LPS-induced TLR4 signaling and pro-inflammatory responses in macrophages and monocytes. Biochem Biophys Res Commun. 2019;508:169–76.
Marongiu L, Gornati L, Artuso I, Zanoni I, Granucci F. Below the surface: the inner lives of TLR4 and TLR9. J Leukoc Biol. 2019;106:147–60.
Park BS, Song DH, Kim HM, Choi BS, Lee H, Lee JO. The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature. 2009;458:1191–5.
Rosadini CV, Kagan JC. Early innate immune responses to bacterial LPS. Curr Opin Immunol. 2017;44:14–19.
Khaled AR, Butfiloski EJ, Sobel ES, Schiffenbauer J. Functional consequences of the SHP-1 defect in motheaten viable mice: role of NF-kappa B. Cell Immunol. 1998;185:49–58.
Massa PT, Wu C. Increased inducible activation of NF-kappaB and responsive genes in astrocytes deficient in the protein tyrosine phosphatase SHP-1. J Interferon Cytokine Res. 1998;18:499–507.
Fujioka Y, Matozaki T, Noguchi T, Iwamatsu A, Yamao T, Takahashi N, et al. A novel membrane glycoprotein, SHPS-1, that binds the SH2-domain-containing protein tyrosine phosphatase SHP-2 in response to mitogens and cell adhesion. Mol Cell Biol. 1996;16:6887–99.
Oshima K, Ruhul Amin AR, Suzuki A, Hamaguchi M, Matsuda S. SHPS-1, a multifunctional transmembrane glycoprotein. FEBS Lett. 2002;519:1–7.
Timms JF, Carlberg K, Gu H, Chen H, Kamatkar S, Nadler MJ, et al. Identification of major binding proteins and substrates for the SH2-containing protein tyrosine phosphatase SHP-1 in macrophages. Mol Cell Biol. 1998;18:3838–50.
Miyake A, Murata Y, Okazawa H, Ikeda H, Niwayama Y, Ohnishi H, et al. Negative regulation by SHPS-1 of Toll-like receptor-dependent proinflammatory cytokine production in macrophages. Genes Cells. 2008;13:209–19.
Lu R, Pan H, Shively JE. CEACAM1 negatively regulates IL-1β production in LPS activated neutrophils by recruiting SHP-1 to a SYK-TLR4-CEACAM1 complex. PLoS Pathog. 2012;8:e1002597.
Ramachandran IR, Song W, Lapteva N, Seethammagari M, Slawin KM, Spencer DM, et al. The phosphatase SRC homology region 2 domain-containing phosphatase-1 is an intrinsic central regulator of dendritic cell function. J Immunol. 2011;186:3934–45.
You M, Flick LM, Yu D, Feng GS. Modulation of the nuclear factor kappa B pathway by Shp-2 tyrosine phosphatase in mediating the induction of interleukin (IL)-6 by IL-1 or tumor necrosis factor. J Exp Med. 2001;193:101–10.
Feng J, He L, Li Y, Xiao F, Hu G. Modeling of PH domains and phosphoinositides interactions and beyond. Adv Exp Med Biol. 2019;1111:19–32.
Niogret C, Birchmeier W, Guarda G. SHP-2 in lymphocytes’ cytokine and inhibitory receptor signaling. Front Immunol. 2019;10:2468.
van Beijnum JR, Buurman WA, Griffioen AW. Convergence and amplification of toll-like receptor (TLR) and receptor for advanced glycation end products (RAGE) signaling pathways via high mobility group B1 (HMGB1). Angiogenesis. 2008;11:91–99.
Buwitt-Beckmann U, Heine H, Wiesmüller KH, Jung G, Brock R, Akira S, et al. Toll-like receptor 6-independent signaling by diacylated lipopeptides. Eur J Immunol. 2005;35:282–9.
Pashenkov MV, Murugina NE, Budikhina AS, Pinegin BV. Synergistic interactions between NOD receptors and TLRs: Mechanisms and clinical implications. J Leukoc Biol. 2019;105:669–80.
Jakopin Ž, Corsini E. THP-1 Cells and pro-inflammatory cytokine production: an in vitro tool for functional characterization of NOD1/NOD2 antagonists. Int J Mol Sci. 2019;20:4265.
van Heel DA, Ghosh S, Butler M, Hunt KA, Lundberg AM, Ahmad T, et al. Muramyl dipeptide and toll-like receptor sensitivity in NOD2-associated Crohn’s disease. Lancet. 2005;365:1794–6.
Bretz NP, Ridinger J, Rupp AK, Rimbach K, Keller S, Rupp C, et al. Body fluid exosomes promote secretion of inflammatory cytokines in monocytic cells via Toll-like receptor signaling. J Biol Chem. 2013;288:36691–702.
Chow A, Zhou W, Liu L, Fong MY, Champer J, Van Haute D, et al. Macrophage immunomodulation by breast cancer-derived exosomes requires Toll-like receptor 2-mediated activation of NF-κB. Sci Rep. 2014;4:5750.
Kim EJ, Suk K, Lee WH. SHPS-1 and a synthetic peptide representing its ITIM inhibit the MyD88, but not TRIF, pathway of TLR signaling through activation of SHP and PI3K in THP-1 cells. Inflamm Res. 2013;62:377–86.
Medvedev AE, Piao W, Shoenfelt J, Rhee SH, Chen H, Basu S, et al. Role of TLR4 tyrosine phosphorylation in signal transduction and endotoxin tolerance. J Biol Chem. 2007;282:16042–53.
Chaudhary A, Fresquez TM, Naranjo MJ. Tyrosine kinase Syk associates with toll-like receptor 4 and regulates signaling in human monocytic cells. Immunol Cell Biol. 2007;85:249–56.
An H, Zhao W, Hou J, Zhang Y, Xie Y, Zheng Y, et al. SHP-2 phosphatase negatively regulates the TRIF adaptor protein-dependent type I interferon and proinflammatory cytokine production. Immunity. 2006;25:919–28.
Shimamura S, Sasaki K, Tanaka M. The Src substrate SKAP2 regulates actin assembly by interacting with WAVE2 and cortactin proteins. J Biol Chem. 2013;288:1171–83.
Fløyel T, Meyerovich K, Prause MC, Kaur S, Frørup C, Mortensen HB, et al. SKAP2, a candidate gene for type 1 diabetes, regulates β-cell apoptosis and glycemic control in newly diagnosed patients. Diabetes. 2021;70:464–76.
Bui TM, Wiesolek HL, Sumagin R. ICAM-1: A master regulator of cellular responses in inflammation, injury resolution, and tumorigenesis. J Leukoc Biol. 2020;108:787–99.
Chen R. Isolation and culture of mouse bone marrow-derived macrophages (BMM’phi'). Bio-protocol. 2012;2:e68.
Acknowledgements
We thank members of the Bioscience Education and Research Support Center (BERSC) of Akita University for technical assistance. This work was supported by JSPS KAKENHI grants (19H03495 to M. Tanaka, 19K07681 to G. Itoh, 18K07192 to S. Kuriyama, 20H01094 to K. Takagane, and 19K07454 to A. Goto), Takeda Science Foundation grants (to M. Tanaka and G. Itoh), a Research Grant from the Princess Takamatsu Cancer Research Fund (19-25123 to M. Tanaka), Smoking Research Foundation to A. Goto, and the Cooperative Research Project Program of Joint Usage/Research Center at the Institute of Development, Aging and Cancer, Tohoku University (G. Itoh).
Funding
This work was supported by JSPS KAKENHI grants (19H03495 to M. Tanaka, 19K07681 to G. Itoh, 21K07090 to S. Kuriyama, 20H01094 to K. Takagane, and 19K07454 to A. Goto), the Takeda Science Foundation grants (to M. Tanaka and G. Itoh), and a Research Grant from the Princess Takamatsu Cancer Research Fund (19-25123 to M. Tanaka).
Author information
Authors and Affiliations
Contributions
KT: data acquisition, data analysis, manuscript writing; MU, GI, SK: experimental methods, data acquisition; AG: data analysis; MT: funding, supervision, experimental design, data analysis, manuscript writing
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Takagane, K., Umakoshi, M., Itoh, G. et al. SKAP2 suppresses inflammation-mediated tumorigenesis by regulating SHP-1 and SHP-2. Oncogene 41, 1087–1099 (2022). https://doi.org/10.1038/s41388-021-02153-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-021-02153-1
This article is cited by
-
Mechanism study of tyrosine phosphatase shp-1 in inhibiting hepatocellular carcinoma progression by regulating the SHP2/GM-CSF pathway in TAMs
Scientific Reports (2024)
-
Synergistic effects of BTN3A1, SHP2, CD274, and STAT3 gene polymorphisms on the risk of systemic lupus erythematosus: a multifactorial dimensional reduction analysis
Clinical Rheumatology (2024)
-
The role of CXCL family members in different diseases
Cell Death Discovery (2023)