Abstract
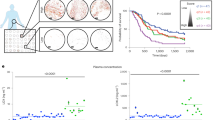
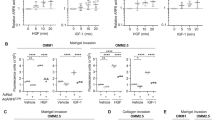
Uveal melanoma (UM) is the most prevalent primary intraocular malignancy in adults, and patients that develop metastases (~50%) survive <1 year, highlighting the urgent need for new therapies. TCGA has recently revealed that a hypoxia gene signature is associated with poor UM patient prognosis. Here we show that expression of hypoxia-regulated collagen prolyl-4-hydroxylase genes P4HA1 and P4HA2 is significantly upregulated in UM patients with metastatic disease and correlates with poor prognosis, suggesting these enzymes might be key tumor drivers. We targeted hypoxia-induced expression of P4HA1/2 in UM with KCN1, a hypoxia inducible factor-1 (HIF-1) pathway inhibitor and found potent inhibition of primary and metastatic disease and extension of animal survival, without overt side effects. At the molecular level, KCN1 antagonized hypoxia-induced expression of P4HA1 and P4HA2, which regulate collagen maturation and deposition in the extracellular matrix. The treatment decreased prolyl hydroxylation, induced proteolytic cleavage and rendered a disordered structure to collagen VI, the main collagen produced by UM, and reduced UM cell invasion. Together, these data demonstrate that extracellular collagen matrix formation can be targeted in UM by inhibiting hypoxia-induced P4HA1 and P4HA2 expression, warranting further development of this strategy in patients with uveal melanoma.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29.
Harbour JW, Chao DL. A molecular revolution in uveal melanoma: implications for patient care and targeted therapy. Ophthalmology. 2014;121:1281–8.
Chattopadhyay C, Kim DW, Gombos DS, Oba J, Qin Y, Williams MD, et al. Uveal melanoma: from diagnosis to treatment and the science in between. Cancer. 2016;122:2299–312.
Dogrusoz M, Jager MJ. Genetic prognostication in uveal melanoma. Acta Ophthalmol. 2018;96:331–47.
Sullivan RJ, Flaherty KT. New strategies in melanoma: entering the era of combinatorial therapy. Clinical cancer research: an official journal of the American Association for. Cancer Res. 2015;21:2424–35.
Klein CA. Cancer. The metastasis cascade. Science. 2008;321:1785–7.
Chiang AC, Massague J. Molecular basis of metastasis. N Engl J Med. 2008;359:2814–23.
Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9:239–52.
Rankin EB, Giaccia AJ. Hypoxic control of metastasis. Science. 2016;352:175–80.
Robertson AG, Shih J, Yau C, Gibb EA, Oba J, Mungall KL, et al. Integrative Analysis Identifies Four Molecular and Clinical Subsets in Uveal Melanoma. Cancer Cell. 2017;32:204–20. e215
Mouriaux F, Sanschagrin F, Diorio C, Landreville S, Comoz F, Petit E, et al. Increased HIF-1alpha expression correlates with cell proliferation and vascular markers CD31 and VEGF-A in uveal melanoma. Investig Ophthalmol Vis Sci. 2014;55:1277–83.
Asnaghi L, Lin MH, Lim KS, Lim KJ, Tripathy A, Wendeborn M, et al. Hypoxia promotes uveal melanoma invasion through enhanced Notch and MAPK activation. PLoS ONE. 2014;9:e105372.
Hu K, Babapoor-Farrokhran S, Rodrigues M, Deshpande M, Puchner B, Kashiwabuchi F, et al. Hypoxia-inducible factor 1 upregulation of both VEGF and ANGPTL4 is required to promote the angiogenic phenotype in uveal melanoma. Oncotarget. 2016;7:7816–28.
el Filali M, Missotten GS, Maat W, Ly LV, Luyten GP, van der Velden PA, et al. Regulation of VEGF-A in uveal melanoma. Investig Ophthalmol Vis Sci. 2010;51:2329–37.
Provenzano PP, Eliceiri KW, Campbell JM, Inman DR, White JG, Keely PJ. Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Med. 2006;4:38.
Canty EG, Kadler KE. Procollagen trafficking, processing and fibrillogenesis. J Cell Sci. 2005;118:1341–53.
Gilkes DM, Chaturvedi P, Bajpai S, Wong CC, Wei H, Pitcairn S, et al. Collagen prolyl hydroxylases are essential for breast cancer metastasis. Cancer Res. 2013;73:3285–96.
Xiong G, Deng L, Zhu J, Rychahou PG, Xu R. Prolyl-4-hydroxylase alpha subunit 2 promotes breast cancer progression and metastasis by regulating collagen deposition. BMC Cancer. 2014;14:1.
Walker C, Mojares E, Del Rio Hernandez A. Role of Extracellular Matrix in Development and Cancer Progression. Int J Mol Sci. 2018 Oct 4;19:3028.
Mun J, Jabbar AA, Devi NS, Liu Y, Van Meir EG, Goodman MM. Structure–activity relationship of 2,2-dimethyl-2H-chromene based arylsulfonamide analogs of 3,4-dimethoxy-N-[(2,2-dimethyl-2H-chromen-6-yl)methyl]-N-phenylbenzenesulfonamide, a novel small molecule hypoxia inducible factor-1 (HIF-1) pathway inhibitor and anti-cancer agent. Bioorg Med Chem. 2012;20:4590–7.
Mooring SR, Jin H, Devi NS, Jabbar AA, Kaluz S, Liu Y, et al. Design and Synthesis of Novel Small-Molecule Inhibitors of the Hypoxia Inducible Factor Pathway. J Med Chem. 2011;54:8471–89.
Shi Q, Yin S, Kaluz S, Ni N, Devi NS, Mun J, et al. Binding Model for the Interaction of Anticancer Arylsulfonamides with the p300 Transcription Cofactor. ACS Med Chem Lett. 2012;3:620–5.
Yin S, Kaluz S, Devi NS, Jabbar AA, de Noronha RG, Mun J, et al. Arylsulfonamide KCN1 Inhibits In Vivo Glioma Growth and Interferes with HIF Signaling by Disrupting HIF-1 alpha Interaction with Cofactors p300/CBP. Clin Cancer Res. 2012;18:6623–33.
Wang W, Ao L, Rayburn ER, Xu H, Zhang X, Zhang X et al. KCN1, a Novel Synthetic Sulfonamide Anticancer Agent: in Vitro and In Vivo Anti-Pancreatic Cancer Activities and Preclinical Pharmacology. PLoS ONE.2012;7:e44883.
Filleur S, Nelius T, de Riese W, Kennedy RC. Characterization of PEDF: a multi-functional serpin family protein. J Cell Biochem. 2009;106:769–75.
Lattier JM, Yang H, Crawford S, Grossniklaus HE. Host pigment epithelium-derived factor (PEDF) prevents progression of liver metastasis in a mouse model of uveal melanoma. Clin Exp Metastasis. 2013;30:969–76.
Dai X, Kaluz S, Jiang Y, Shi L, McKinley D, Wang Y, et al. A novel small-molecule arylsulfonamide causes energetic stress and suppresses breast and lung tumor growth and metastasis. Oncotarget. 2017;8:99245–60.
Gelderblom H, Verweij J, Nooter K, Sparreboom A, Cremophor EL. The drawbacks and advantages of vehicle selection for drug formulation. Eur J Cancer. 2001;37:1590–8.
Chen P, Cescon M, Bonaldo P. Collagen VI in cancer and its biological mechanisms. Trends Mol Med. 2013;19:410–7.
Diener-West M, Earle JD, Fine SL, Hawkins BS, Moy CS, Reynolds SM, et al. The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma, III: initial mortality findings. COMS Report No. 18. Arch Ophthalmol. 2001;119:969–82.
Ferguson JH, De Los Santos Z, Devi SN, Kaluz S, Van Meir EG, Zingales SK, et al. Design and synthesis of benzopyran-based inhibitors of the hypoxia-inducible factor-1 pathway with improved water solubility. J Enzym Inhib Med Chem. 2017;32:992–1001.
Tan C, de Noronha RG, Devi NS, Jabbar AA, Kaluz S, Liu Y, et al. Sulfonamides as a new scaffold for hypoxia inducible factor pathway inhibitors. Bioorg Med Chem Lett. 2011;21:5528–32.
Dong L, You S, Zhang Q, Osuka S, Devi NS, Kaluz S, et al. Arylsulfonamide 64B Inhibits Hypoxia/HIF-Induced Expression of c-Met and CXCR4 and Reduces Primary Tumor Growth and Metastasis of Uveal Melanoma. Clin Cancer Res. 2019;25:2206–18.
Hofbauer KH, Gess B, Lohaus C, Meyer HE, Katschinski D, Kurtz A. Oxygen tension regulates the expression of a group of procollagen hydroxylases. Eur J Biochem. 2003;270:4515–22.
Grimmer C, Balbus N, Lang U, Aigner T, Cramer T, Muller L, et al. Regulation of type II collagen synthesis during osteoarthritis by prolyl-4-hydroxylases: possible influence of low oxygen levels. Am J Pathol. 2006;169:491–502.
Stegen S, Laperre K, Eelen G, Rinaldi G, Fraisl P, Torrekens S, et al. HIF-1alpha metabolically controls collagen synthesis and modification in chondrocytes. Nature. 2019;565:511–5.
Gilkes DM, Bajpai S, Chaturvedi P, Wirtz D, Semenza GL. Hypoxia-inducible factor 1 (HIF-1) promotes extracellular matrix remodeling under hypoxic conditions by inducing P4HA1, P4HA2, and PLOD2 expression in fibroblasts. J Biol Chem. 2013;288:10819–29.
Onken MD, Worley LA, Davila RM, Char DH, Harbour JW. Prognostic testing in uveal melanoma by transcriptomic profiling of fine needle biopsy specimens. J Mol Diagn. 2006;8:567–73.
Zhang Q, Yang H, Kang SJ, Wang Y, Wang GD, Coulthard T, et al. In vivo high-frequency, contrast-enhanced ultrasonography of uveal melanoma in mice: imaging features and histopathologic correlations. Investig Ophthalmol Vis Sci. 2011;52:2662–8.
Gangemi R, Mirisola V, Barisione G, Fabbi M, Brizzolara A, Lanza F, et al. Mda-9/syntenin is expressed in uveal melanoma and correlates with metastatic progression. PloS ONE. 2012;7:e29989.
Laurent C, Valet F, Planque N, Silveri L, Maacha S, Anezo O, et al. High PTP4A3 phosphatase expression correlates with metastatic risk in uveal melanoma patients. Cancer Res. 2011;71:666–74.
Goswami CP, Nakshatri H. PROGgeneV2: enhancements on the existing database. BMC Cancer. 2014;14:970.
Robinson JT, Thorvaldsdottir H, Winckler W, Guttman M, Lander ES, Getz G, et al. Integrative genomics viewer. Nat Biotechnol. 2011;29:24–26.
Van Meir EG. Identification of nude mice in tumorigenicity assays. Int J Cancer. 1997;71:310.
Acknowledgements
We thank Dr Bruce Ksander (Schepens Eye Institute, Boston, MA) for providing Mel290 and OMM2-5 cells (OMM2-5 was established by Dr Timothy Murray, Bascom Palmer Eye Institute, Miami, FL); Dr Jerry Niederkorn (Department of Ophthalmology, UT Southwestern, Dallas, TX) for OMM1 and 92.1 cells (92.1 cells were established by Dr Gregorius P. Luyten, Department of Ophthalmology, Rotterdam University Hospital); Dr June Kan-Mitchel (Wayne State University, Detroit, MI) for OCM1 cells, and Dr Dario Rusciano (Friedrich Miescher Institute, Basel, Switzerland) for providing B16LS9 cells. We thank the Winship Research Pathology Core Lab (Emory University) for IHC and Masson’s staining. We also acknowledge Zhengjia Chen (Department of Biostatistics & Bioinformatics, Emory Rollins School of Public Health) for providing statistical consultation. We appreciate the helpful advice and assistance of all members of the Laboratory of Molecular Neuro-Oncology. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby-marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Funding
This work was supported in part by grants from the NIH (R01 CA116804, R01 CA176001, R01 CA180805, R24 EY017045, P30 EY06360, P30 CA138292 and P30 CA13148), the National Natural Science Foundation of China (NNS81201808), the Fight For Sight Postdoctoral Award, the Emory Melanoma Prevention and Research Discovery Fund, a Winship Cancer Institute pilot grant, a Central South University Lieying pilot grant, the V Foundation, the Max Cure Foundation, the Samuel Waxman Cancer Research Foundation, the Alan B. Slifka Foundation, and an unrestricted grant from Research to Prevent Blindness, Inc.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
EGVM is an inventor on a patent jointly held by Emory University and Scripps Research Institute that includes the KCN1 compound used in this study. He is also a share-holder of OncoSpherix Inc.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Kaluz, S., Zhang, Q., Kuranaga, Y. et al. Targeting HIF-activated collagen prolyl 4-hydroxylase expression disrupts collagen deposition and blocks primary and metastatic uveal melanoma growth. Oncogene 40, 5182–5191 (2021). https://doi.org/10.1038/s41388-021-01919-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-021-01919-x