Abstract
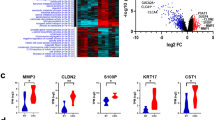
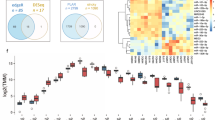
Hepatocellular carcinoma (HCC) is the most common subtype of primary liver cancer and one of the leading causes of cancer-related death worldwide. To gain more insights into the transcriptomic landscape and molecular mechanism of HCC, we performed TMT-labelled tandem mass spectrometry (n = 4) and whole-transcriptome sequencing (n = 3) based on HCC tumour (T) and adjacent normal (N) tissues from seven HCC patients. To comprehensively evaluate the gene-regulatory circuits in HCC, differential expression and enrichment analyses were performed on the differentially expressed proteins (DEPs), genes (DEGs), miRNAs (555), lncRNAs (29) and circRNAs (895). A total of 977 proteins and 243 genes were found to be differentially expressed in HCC tumours compared with adjacent normal tissues. HCC data from The Cancer Genome Atlas were used to validate the results. Combined with the results above, 56 DEP-DEGs with common changes in relative quantity were identified. Functional pathway analysis showed that the DEP-DEGs were mainly enriched in the spliceosome and various metabolic processes. Bioinformatics analysis showed that hsa-miR-1266-5p, hsa-miR-128-1-5p, hsa-miR-139-5p, hsa-miR-34b-3p and hsa-miR-570-3p were involved in the regulation of the hub genes mentioned above. The crucial coexpression (lncRNA–mRNA, circRNA–mRNA) and competing endogenous RNA interaction axes showed the possible functions of the lncRNAs and circRNAs. We explored potential cancer biomarkers by combining proteomic and transcriptomic studies. Our study provides a valuable resource for understanding regulatory mechanisms at the RNA level and may ultimately further assist in the development of diagnostic and/or therapeutic targets for HCC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium (http://www.ebi.ac.uk/pride/archive/) via the PRIDE partner repository with the dataset identifier PXD014915. The RNA-Seq datasets have been uploaded to The National Omics Data Encyclopedia (https://www.biosino.org/node/) with the accession number OEP001672.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
Henley SJ, Ward EM, Scott S, Ma J, Anderson RN, Firth AU, et al. Annual report to the nation on the status of cancer, part I: national cancer statistics. Cancer. 2020;126:2225–49.
Fu J, Wang H. Precision diagnosis and treatment of liver cancer in China. Cancer Lett. 2018;412:283–8.
Sia D, Villanueva A, Friedman SL, Llovet JM. Liver cancer cell of origin, molecular class, and effects on patient prognosis. Gastroenterology. 2017;152:745–61.
Dhanasekaran R, Nault JC, Roberts LR, Zucman-Rossi J. Genomic medicine and implications for hepatocellular carcinoma prevention and therapy. Gastroenterology. 2019;156:492–509.
Jiang Y, Sun A, Zhao Y, Ying W, Sun H, Yang X, et al. Proteomics identifies new therapeutic targets of early-stage hepatocellular carcinoma. Nature. 2019;567:257–61.
Sasaki R, Kanda T, Yokosuka O, Kato N, Matsuoka S, Moriyama M. Exosomes and hepatocellular carcinoma: from bench to bedside. Int J Mol Sci. 2019;20:1406.
Zhao Q, Zhang Z, Li J, Xu F, Zhang B, Liu M, et al. Lysine acetylome study of human hepatocellular carcinoma tissues for biomarkers and therapeutic targets discovery. Front Genet. 2020;11:572663.
Alqahtani A, Khan Z, Alloghbi A, Said Ahmed TS, Ashraf M, Hammouda DM. Hepatocellular carcinoma: molecular mechanisms and targeted therapies. Medicina (Kaunas). 2019;55:526.
Chen S, Cao Q, Wen W, Wang H. Targeted therapy for hepatocellular carcinoma: challenges and opportunities. Cancer Lett. 2019;460:1–9.
Mahipal A, Kommalapati A, Mehta R, Kim RD, Hoshida Y. Molecular-targeted therapies in hepatocellular carcinoma. Hepatocellular carcinoma: translational precision medicine approaches. Cham (CH). humana Press; 2019. P. 225–38.
Umeda S, Kanda M, Kodera Y. Recent advances in molecular biomarkers for patients with hepatocellular carcinoma. Expert Rev Mol Diagn. 2019;19:725–38.
Chen B, Butte AJ. Leveraging big data to transform target selection and drug discovery. Clin Pharm Ther. 2016;99:285–97.
Wooden B, Goossens N, Hoshida Y, Friedman SL. Using big data to discover diagnostics and therapeutics for gastrointestinal and liver diseases. Gastroenterology.2017;152:53–67. e3.
Fujiwara N, Friedman SL, Goossens N, Hoshida Y. Risk factors and prevention of hepatocellular carcinoma in the era of precision medicine. J Hepatol. 2018;68:526–49.
Llovet JM, Montal R, Sia D, Finn RS. Molecular therapies and precision medicine for hepatocellular carcinoma. Nat Rev Clin Oncol. 2018;15:599–616.
Lin YH, Wu MH, Yeh CT, Lin KH. Long non-coding RNAs as mediators of tumor microenvironment and liver cancer cell communication. Int J Mol Sci. 2018;19:3742.
Chen X, Tang FR, Arfuso F, Cai WQ, Ma Z, Yang J, et al. The emerging role of long non-coding RNAs in the metastasis of hepatocellular carcinoma. Biomolecules. 2019;10:66.
Dragomir MP, Kopetz S, Ajani JA, Calin GA. Non-coding RNAs in GI cancers: from cancer hallmarks to clinical utility. Gut. 2020;69:748–63.
El Khodiry A, Afify M, El, Tayebi HM. Behind the curtain of non-coding RNAs; long non-coding RNAs regulating hepatocarcinogenesis. World J Gastroenterol. 2018;24:549–72.
Lim LJ, Wong SYS, Huang F, Lim S, Chong SS, Ooi LL, et al. Roles and regulation of long noncoding RNAs in hepatocellular carcinoma. Cancer Res. 2019;79:5131–9.
Zhang H, Chen X, Zhang J, Wang X, Chen H, Liu L, et al. Long noncoding RNAs in HBVrelated hepatocellular carcinoma (Review). Int J Oncol. 2020;56:18–32.
Huang Z, Zhou JK, Peng Y, He W, Huang C. The role of long noncoding RNAs in hepatocellular carcinoma. Mol Cancer. 2020;19:77.
Kim YA, Park KK, Lee SJ. LncRNAs act as a link between chronic liver disease and hepatocellular carcinoma. Int J Mol Sci. 2020;21:2883.
Wei L, Wang X, Lv L, Liu J, Xing H, Song Y, et al. The emerging role of microRNAs and long noncoding RNAs in drug resistance of hepatocellular carcinoma. Mol Cancer. 2019;18:147.
Yin Y, Long J, He Q, Li Y, Liao Y, He P, et al. Emerging roles of circRNA in formation and progression of cancer. J Cancer. 2019;10:5015–21.
Chen TC, Tallo-Parra M, Cao QM, Kadener S, Bottcher R, Perez-Vilaro G, et al. Host-derived circular RNAs display proviral activities in Hepatitis C virus-infected cells. PLoS Pathog. 2020;16:e1008346.
Afify AY, Ibrahim SA, Aldamsisi MH, Zaghloul MS, El-Ekiaby N, Abdelaziz AI. Competing endogenous RNAs in hepatocellular carcinoma-the pinnacle of rivalry. Semin Liver Dis. 2019;39:463–75.
Xu G, Xu WY, Xiao Y, Jin B, Du SD, Mao YL, et al. The emerging roles of non-coding competing endogenous RNA in hepatocellular carcinoma. Cancer Cell Int. 2020;20:496.
Niu ZS, Wang WH, Dong XN, Tian LM. Role of long noncoding RNA-mediated competing endogenous RNA regulatory network in hepatocellular carcinoma. World J Gastroenterol. 2020;26:4240–60.
Li L, Wang H. Heterogeneity of liver cancer and personalized therapy. Cancer Lett. 2016;379:191–7.
Shi J, Chen L, Chen Y, Lu Y, Chen X, Yang Z. Aldo-Keto Reductase Family 1 Member B10 (AKR1B10) overexpression in tumors predicts worse overall survival in hepatocellular carcinoma. J Cancer. 2019;10:4892–901.
DiStefano JK, Davis B. Diagnostic and prognostic potential of AKR1B10 in human hepatocellular carcinoma. Cancers (Basel). 2019;11:486.
Du Z, Liu X, Wei X, Luo H, Li P, Shi M, et al. Quantitative proteomics identifies a plasma multi-protein model for detection of hepatocellular carcinoma. Sci Rep. 2020;10:15552.
Ye X, Li C, Zu X, Lin M, Liu Q, Liu J, et al. A large-scale multicenter study validates Aldo-Keto Reductase Family 1 Member B10 as a prevalent serum marker for detection of hepatocellular carcinoma. Hepatology. 2019;69:2489–501.
Tseng CS, Tang KS, Lo HW, Ker CG, Teng HC, Huang CS. UDP-glucuronosyltransferase 1A7 genetic polymorphisms are associated with hepatocellular carcinoma risk and onset age. Am J Gastroenterol. 2005;100:1758–63.
Zhao Y, Li M, Yao X, Fei Y, Lin Z, Li Z, et al. HCAR1/MCT1 regulates tumor ferroptosis through the lactate-mediated AMPK-SCD1 activity and its therapeutic implications. Cell Rep. 2020;33:108487.
Yan T, Gao S, Peng X, Shi J, Xie C, Li Q, et al. Significantly decreased and more variable expression of major CYPs and UGTs in liver microsomes prepared from HBV-positive human hepatocellular carcinoma and matched pericarcinomatous tissues determined using an isotope label-free UPLC-MS/MS method. Pharm Res. 2015;32:1141–57.
Nekvindova J, Mrkvicova A, Zubanova V, Hyrslova Vaculova A, Anzenbacher P, Soucek P, et al. Hepatocellular carcinoma: gene expression profiling and regulation of xenobiotic-metabolizing cytochromes P450. Biochem Pharm. 2020;177:113912.
Lanzafame M, Bianco G, Terracciano LM, Ng CKY, Piscuoglio S. The role of long non-coding RNAs in hepatocarcinogenesis. Int J Mol Sci. 2018;19:682.
Mahpour A, Mullen AC. Our emerging understanding of the roles of long non-coding RNAs in normal liver function, disease, and malignancy. JHEP Rep. 2021;3:100177.
Kong S, Tao M, Shen X, Ju S. Translatable circRNAs and lncRNAs: driving mechanisms and functions of their translation products. Cancer Lett. 2020;483:59–65.
He J, Zuo Q, Hu B, Jin H, Wang C, Cheng Z, et al. A novel, liver-specific long noncoding RNA LINC01093 suppresses HCC progression by interaction with IGF2BP1 to facilitate decay of GLI1 mRNA. Cancer Lett. 2019;450:98–109.
Liu Y, Zhao Q, Xu F, Wang K, Zhao Y, Chen H, et al. Dysregulation of phosphoproteins in hepatocellular carcinoma revealed via quantitative analysis of the phosphoproteome. Oncol Lett. 2021;21:117.
Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57.
Huang da W, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37:1–13.
Kohl M, Wiese S, Warscheid B. Cytoscape: software for visualization and analysis of biological networks. Methods Mol Biol. 2011;696:291–303.
Acknowledgements
We gratefully acknowledge Henan Key Laboratory of Pharmacology for Liver Diseases for providing experimental platform support. We thank Dr. Jianxiang Shi for assistance in bioinformatic analysis. This work was supported by grants from Natural Science Foundation of Henan Province (No. 182300410361); the Major Project of Science and Technology in Henan Province (No. 161100311400); the National Science and Technology Major Project of China (No. 2018ZX10302205); Project of Basic Research Fund of Henan Institute of Medical and Pharmacological Sciences (No. 2020BP0107; No. 2020BP0111); Zhengzhou Major Project for Collaborative Innovation (18XTZX12007) and The Key Scientific and Technological Project of Henan Province (No. 212102310124).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Xu, F., Jiang, L., Zhao, Q. et al. Whole-transcriptome and proteome analyses identify key differentially expressed mRNAs, miRNAs, lncRNAs and circRNAs associated with HCC. Oncogene 40, 4820–4831 (2021). https://doi.org/10.1038/s41388-021-01908-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-021-01908-0