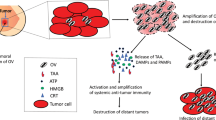
Abstract
Triple-negative breast cancer (TNBC) is the most aggressive molecular subtype among breast tumors and remains a challenge even for the most current therapeutic regimes. Here, we demonstrate that oncolytic alphavirus M1 effectively kills both TNBC and non-TNBC. ER-stress and apoptosis pathways are responsible for the cell death in non-TNBC as reported in other cancer types, yet the cell death in TNBC does not depend on these pathways. Transcriptomic analysis reveals that the M1 virus activates necroptosis in TNBC, which can be pharmacologically blocked by necroptosis inhibitors. By screening a library of clinically available compounds commonly used for breast cancer treatment, we find that Doxorubicin enhances the oncolytic effect of the M1 virus by up to 100-fold specifically in TNBC in vitro, and significantly stalls the tumor growth of TNBC in vivo, through promoting intratumoral virus replication and further triggering apoptosis in addition to necroptosis. These findings reveal a novel antitumor mechanism and a new combination regimen of the M1 oncolytic virus in TNBC, and highlight a need to bridge molecular diagnosis with virotherapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7-30.
DeSantis CE, Ma J, Goding Sauer A, Newman LA, Jemal A. Breast cancer statistics, 2017, racial disparity in mortality by state. CA Cancer J Clin. 2017;67:439-448.
Dent R, Hanna WM, Trudeau M, Rawlinson E, Sun P, Narod SA. Pattern of metastatic spread in triple-negative breast cancer. Breast Cancer Res Treat. 2009;115:423–8.
Wahba HA, El-Hadaad HA. Current approaches in treatment of triple-negative breast cancer. Cancer Biol Med. 2015;12:106-16.
Adair RA, Roulstone V, Scott KJ, Morgan R, Nuovo GJ, Fuller M, et al. Cell carriage, delivery, and selective replication of an oncolytic virus in tumor in patients. Sci Transl Med. 2012;4:138–77.
Miest TS, Cattaneo R. New viruses for cancer therapy: meeting clinical needs. Nat Rev Microbiol. 2014;12:23–34.
Cai J, Yan G. The identification and development of a novel oncolytic virus: alphavirus M1. Hum Gene Ther. 2021;32:138–49.
Rehman H, Silk AW, Kane MP, Kaufman HL. Into the clinic: talimogene laherparepvec (T-VEC), a first-in-class intratumoral oncolytic viral therapy. J Immunother Cancer. 2016;4:53.
Poh A. First oncolytic viral therapy for melanoma. Cancer Discov. 2016;6:6.
Russell SJ, Peng K-W, Bell JC. Oncolytic virotherapy. Nat Biotechnol. 2012;30:658–70.
Lawler SE, Speranza M-C, Cho C-F, Chiocca EA. Oncolytic viruses in cancer treatment: a review. JAMA Oncol. 2017;3:841–9.
Goradel NH, Baker AT, Arashkia A, Ebrahimi N, Ghorghanlu S, et al. Oncolytic virotherapy: Challenges and solutions. Curr Probl Cancer. 2021;45:100639.
Moskovskich A, Goldmann U, Kartnig F, Lindinger S, Konecka J, Fiume G, et al. The transporters SLC35A1 and SLC30A1 play opposite roles in cell survival upon VSV virus infection. Sci Rep. 2019;9:10471.
de Graaf JF, de Vor L, Fouchier RAM, van den Hoogen BG. Armed oncolytic viruses: a kick-start for anti-tumor immunity. Cytokine Growth Factor Rev. 2018;41:28–39.
Turnbull S, West EJ, Scott KJ, Appleton E, Melcher A, Ralph C. Evidence for oncolytic virotherapy: where have we got to and where are we going? Viruses. 2015;7:6291–312.
Wen J-S, Zhao W-Z, Liu J-W, Zhou H, Tao J-P, Yan H-J, et al. Genomic analysis of a Chinese isolate of Getah-like virus and its phylogenetic relationship with other Alphaviruses. Virus Genes. 2007;35:597–603.
Brown CM, Timoney PJ. Getah virus infection of Indian horses. Trop Anim Health Prod. 1998;30:241–52.
Zhang H, Lin Y, Li K, Liang J, Xiao X, et al. Naturally Existing Oncolytic Virus M1 Is Nonpathogenic for the Nonhuman Primates After Multiple Rounds of Repeated Intravenous Injections. Hum Gene Ther. 2016;27:700-11.
Pu Z-Q, Liu D, Lobo Mouguegue HPP, Jin C-W, Sadiq E, Qin D-D, et al. NR4A1 counteracts JNK activation incurred by ER stress or ROS in pancreatic β-cells for protection. J Cell Mol Med. 2020;24:14171–83.
Tan Y, Lin Y, Li K, Xiao X, Liang J, Cai J, et al. Selective antagonism of Bcl-xL potentiates M1 oncolysis by enhancing mitochondrial apoptosis. Hum Gene Ther. 2018;29:950–61.
Zhang H, Li K, Lin Y, Xing F, Xiao X, et al. Targeting VCP enhances anticancer activity of oncolytic virus M1 in hepatocellular carcinoma. Sci Transl Med. 2017;9:eaam7996.
Xiao X, Liang J, Huang C, Li K, Xing F, Zhu W, et al. DNA-PK inhibition synergizes with oncolytic virus M1 by inhibiting antiviral response and potentiating DNA damage. Nat Commun. 2018;9:4342.
Muhuri M, Gao G. Oncolytic virus alphavirus M1: a new and promising weapon to fight cancer. Hum Gene Ther 2021;32:136–7.
Hu J, Cai X-F, Yan G. Alphavirus M1 induces apoptosis of malignant glioma cells via downregulation and nucleolar translocation of p21WAF1/CIP1 protein. Cell Cycle. 2009;8:3328–39.
Lin Y, Zhang H, Liang J, Li K, Zhu W, Fu L, et al. Identification and characterization of alphavirus M1 as a selective oncolytic virus targeting ZAP-defective human cancers. Proc Natl Acad Sci USA. 2014;111:E4504–12.
Sun L, Wang H, Wang Z, He S, Chen S, Liao D, et al. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell. 2012;148:213–27.
Gong Y, Fan Z, Luo G, Yang C, Huang Q. et al. The role of necroptosis in cancer biology and therapy. Mol Cancer. 2019;18:100 https://doi.org/10.1186/s12943-019-1029-8.
Zhang J, Yang Y, He W, Sun L. Necrosome core machinery: MLKL. Cell Mol Life Sci. 2016;73:2153–63.
Tanzer MC, Matti I, Hildebrand JM, Young SN, Wardak A, Tripaydonis A, et al. Evolutionary divergence of the necroptosis effector MLKL. Cell Death Differ. 2016;23:1185–97.
Liu S, Liu H, Johnston A, Hanna-Addams S, Reynoso E, Xiang Y, et al. MLKL forms disulfide bond-dependent amyloid-like polymers to induce necroptosis. Proc Natl Acad Sci USA. 2017;114:E7450–9.
Budhwani M, Mazzieri R, Dolcetti R. Plasticity of type I interferon-mediated responses in cancer therapy: from anti-tumor immunity to resistance. Front Oncol. 2018;8:322.
Lazear HM, Schoggins JW, Diamond MS. Shared and distinct functions of type I and type III interferons. Immunity. 2019;50:907–23.
Kok F, Rosenblatt M, Teusel M, Nizharadze T, Gonçalves Magalhães V, Dächert C, et al. Disentangling molecular mechanisms regulating sensitization of interferon alpha signal transduction. Mol Syst Biol. 2020;16:e8955.
Willson J. A matter of life and death for caspase 8. Nat Rev Mol Cell Biol. 2020;21:63.
Silke J, Strasser A. The FLIP Side of Life. Sci Signal. 2013;15;6:pe2.
Vanlangenakker N, Berghe T Vanden, Bogaert P, Laukens B, Zobel K, Deshayes K, et al. cIAP1 and TAK1 protect cells from TNF-induced necrosis by preventing RIP1/RIP3-dependent reactive oxygen species production. Cell Death Differ. 2011;656–65.
Wu J, Mamidi TKK, Zhang L, Hicks C. Unraveling the genomic-epigenomic interaction landscape in triple negative and non-triple negative breast cancer. Cancers. 2020;12. https://doi.org/10.3390/cancers12061559.
Thorn CF, Oshiro C, Marsh S, Hernandez-Boussard T, McLeod H, et al. Doxorubicin pathways: pharmacodynamics and adverse effects. Pharmacogenet Genomics. 2011;21:440-6.
Forrest RA, Swift LP, Rephaeli A, Nudelman A, Kimura K-I, Phillips DR, et al. Activation of DNA damage response pathways as a consequence of anthracycline-DNA adduct formation. Biochem Pharm. 2012;83:1602–12.
Wen S-H, Su S-C, Liou B-H, Lin C-H, Lee K-R. Sulbactam-enhanced cytotoxicity of doxorubicin in breast cancer cells. Cancer Cell Int. 2018;18:128.
Pang B, Qiao X, Janssen L, Velds A, Groothuis T, Kerkhoven R, et al. Drug-induced histone eviction from open chromatin contributes to the chemotherapeutic effects of doxorubicin. Nat Commun. 2013;4:1908.
Yang F, Teves SS, Kemp CJ, Henikoff S. Doxorubicin, DNA torsion, and chromatin dynamics. Biochim Biophys Acta. 2014;1845:84–89.
Yang F, Kemp CJ, Henikoff S. Doxorubicin enhances nucleosome turnover around promoters. Curr Biol. 2013;23:782–7.
Garrido-Castro AC, Lin NU, Polyak K. Insights into molecular classifications of triple-negative breast cancer: improving patient selection for treatment. Cancer Discov. 2019;9:176–98.
Zhu W, Liang J, Tan J, Guo L, Cai J, Hu J, et al. Real-time visualization and quantification of oncolytic M1 virus in vitro and in vivo. Hum Gene Ther. 2021;32:158–65.
Jensen EC. Quantitative analysis of histological staining and fluorescence using ImageJ. Anat Rec. 2013;296:378–81.
Trapnell C, Pachter, L, Salzberg SL. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics. 2009;25:1105–11.
Anders S, Pyl PT, Huber W. HTSeq—a Python framework to work with high-throughput sequencing data. Bioinformatics. 2015;31:166–9.
Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550.
Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102:15545–50.
Reimand J, Isserlin R, Voisin V, Kucera M, Tannus-Lopes C, Rostamianfar A, et al. Pathway enrichment analysis and visualization of omics data using g:Profiler, GSEA, Cytoscape and EnrichmentMap. Nat Protoc. 2019;14:482–517.
Hänzelmann S, Castelo R, Guinney J. GSVA: gene set variation analysis for microarray and RNA-seq data. BMC Bioinform. 2013;14:7.
Acknowledgements
We thank all authors who contributed to this work. This work was supported by the National Natural Science Foundation of China (No. 81872886), Fundamental Research Funds for the Central Universities (19ykpy36 and 19ykpy166), Guangdong Basic and Applied Basic Research Foundation (2020A1515011446), Pioneering talents project of Guangzhou Development Zone, Guangdong Province (CY2018-012), and Leading Team for Entrepreneurship in Guangzhou, Guangdong Province (201809020004).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, J., Liu, Y., Tan, J. et al. Necroptotic virotherapy of oncolytic alphavirus M1 cooperated with Doxorubicin displays promising therapeutic efficacy in TNBC. Oncogene 40, 4783–4795 (2021). https://doi.org/10.1038/s41388-021-01869-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-021-01869-4
This article is cited by
-
Directed natural evolution generates a next-generation oncolytic virus with a high potency and safety profile
Nature Communications (2023)
-
Coxsackievirus A11 is an immunostimulatory oncolytic virus that induces complete tumor regression in a human non-small cell lung cancer
Scientific Reports (2023)
-
SOCS3 inhibiting JAK-STAT pathway enhances oncolytic adenovirus efficacy by potentiating viral replication and T-cell activation
Cancer Gene Therapy (2023)
-
Oncolytic virotherapy: basic principles, recent advances and future directions
Signal Transduction and Targeted Therapy (2023)
-
Targeting regulated cell death (RCD) with small-molecule compounds in triple-negative breast cancer: a revisited perspective from molecular mechanisms to targeted therapies
Journal of Hematology & Oncology (2022)