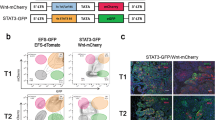
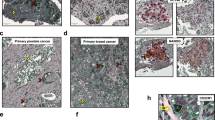
Abstract
Recent findings suggest that the dissemination of tumor cells occurs at the early stage of breast and pancreatic carcinogenesis, which is known as early dissemination. The evidence of early dissemination has been demonstrated predominantly in the bloodstream and bone marrow; however, limited evidence has revealed the existence and behavior of disseminated cells in distant organs. Here, we show that premalignant pancreatic cells seed distant stealth metastasis that eventually develops into manifest metastasis. By analyzing lineage-labeled pancreatic cancer mouse models (KPCT/TFF1KO; Pdx1-Cre/LSL-KRASG12D/LSL-p53R172H/LSL-tdTomato/TFF1KO), we found that premalignant pancreatic cells, rather than mature malignant cells, were prone to enter the bloodstream and reside in the bone marrow, liver, and lung. While these metastatic cells exhibited the characteristics of the cells of host organs and did not behave as malignant cells, they underwent malignant transformation and formed distinct tumors. Surprisingly, the manifestation of distant metastasis occurred even before tumor development in the primary site. Our data revealed that disseminated premalignant cells reside stealthily in distant organs and evolve in parallel with the progression of the primary tumor. These observations suggest that we must rebuild a therapeutic strategy for metastatic pancreatic cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Harper KL, Sosa MS, Entenberg D, Hosseini H, Cheung JF, Nobre R, et al. Mechanism of early dissemination and metastasis in Her2(+) mammary cancer. Nature. 2016;540:588–92.
Hosseini H, Obradovic MMS, Hoffmann M, Harper KL, Sosa MS, Werner-Klein M, et al. Early dissemination seeds metastasis in breast cancer. Nature. 2016;540:552–8.
Husemann Y, Geigl JB, Schubert F, Musiani P, Meyer M, Burghart E, et al. Systemic spread is an early step in breast cancer. Cancer Cell. 2008;13:58–68.
Rhim AD, Mirek ET, Aiello NM, Maitra A, Bailey JM, McAllister F, et al. EMT and dissemination precede pancreatic tumor formation. Cell. 2012;148:349–61.
Banys M, Gruber I, Krawczyk N, Becker S, Kurth R, Wallwiener D, et al. Hematogenous and lymphatic tumor cell dissemination may be detected in patients diagnosed with ductal carcinoma in situ of the breast. Breast Cancer Res Treat. 2012;131:801–8.
Klein CA, Blankenstein TJF, Schmidt-Kittler O, Petronio M, Polzer B, Stoecklein NH, et al. Genetic heterogeneity of single disseminated tumour cells in minimal residual cancer. Lancet. 2002;360:683–9.
Sanger N, Effenberger KE, Riethdorf S, Van Haasteren V, Gauwerky J, Wiegratz I, et al. Disseminated tumor cells in the bone marrow of patients with ductal carcinoma in situ. Int J Cancer. 2011;129:2522–6.
Rhim AD, Thege FI, Santana SM, Lannin TB, Saha TN, Tsai S, et al. Detection of circulating pancreas epithelial cells in patients with pancreatic cystic lesions. Gastroenterology. 2014;146:647–51.
Klein CA. Parallel progression of primary tumours and metastases. Nat Rev Cancer. 2009;9:302–12.
Giancotti FG. Mechanisms governing metastatic dormancy and reactivation. Cell. 2013;155:750–64.
Sosa MS, Bragado P, Aguirre-Ghiso JA. Mechanisms of disseminated cancer cell dormancy: an awakening field. Nat Rev Cancer. 2014;14:611–22.
Ochiai Y, Yamaguchi J, Kokuryo T, Yokoyama Y, Ebata T, Nagino M. Trefoil factor family 1 inhibits the development of hepatocellular carcinoma by regulating beta-catenin activation. Hepatology. 2020;72:503–17.
Soutto M, Peng D, Katsha A, Chen Z, Piazuelo MB, Washington MK, et al. Activation of beta-catenin signalling by TFF1 loss promotes cell proliferation and gastric tumorigenesis. Gut. 2015;64:1028–39.
Hingorani SR, Petricoin EF, Maitra A, Rajapakse V, King C, Jacobetz MA, et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4:437–50.
Morton JP, Timpson P, Karim SA, Ridgway RA, Athineos D, Doyle B, et al. Mutant p53 drives metastasis and overcomes growth arrest/senescence in pancreatic cancer. Proc Natl Acad Sci USA. 2010;107:246–51.
Yamaguchi J, Yokoyama Y, Kokuryo T, Ebata T, Enomoto A, Nagino M. Trefoil factor 1 inhibits epithelial-mesenchymal transition of pancreatic intraepithelial neoplasm. J Clin Invest. 2018;128:3619–29.
Sunagawa M, Yamaguchi J, Kokuryo T, Ebata T, Yokoyama Y, Sugawara G, et al. Trefoil factor family 1 expression in the invasion front is a poor prognostic factor associated with lymph node metastasis in pancreatic cancer. Pancreatology. 2017;17:782–7.
Kurashige M, Kohara M, Ohshima K, Tahara S, Hori Y, Nojima S, et al. Origin of cancer-associated fibroblasts and tumor-associated macrophages in humans after sex-mismatched bone marrow transplantation. Commun Biol. 2018;1:131.
Wang L, Yang H, Abel EV, Ney GM, Palmbos PL, Bednar F, et al. ATDC induces an invasive switch in KRAS-induced pancreatic tumorigenesis. Genes Dev. 2015;29:171–83.
Ao Z, Shah SH, Machlin LM, Parajuli R, Miller PC, Rawal S, et al. Identification of cancer-associated fibroblasts in circulating blood from patients with metastatic breast cancer. Cancer Res. 2015;75:4681–7.
Min S, Xiaoyan X, Fanghui P, Yamei W, Xiaoli Y, Feng W. The glioma-associated oncogene homolog 1 promotes epithelial–mesenchymal transition in human esophageal squamous cell cancer by inhibiting E-cadherin via Snail. Cancer Gene Ther. 2013;20:379–85.
Yatabe Y, Mitsudomi T, Takahashi T. TTF-1 expression in pulmonary adenocarcinomas. Am J Surg Pathol. 2002;26:767–73.
Clawson GA, Matters GL, Xin P, McGovern C, Wafula E, dePamphilis C, et al. “Stealth dissemination” of macrophage-tumor cell fusions cultured from blood of patients with pancreatic ductal adenocarcinoma. PLoS One. 2017;12:e0184451.
Podsypanina K, Du YC, Jechlinger M, Beverly LJ, Hambardzumyan D, Varmus H. Seeding and propagation of untransformed mouse mammary cells in the lung. Science. 2008;321:1841–4.
Fizazi K, Greco FA, Pavlidis N, Daugaard G, Oien K, Pentheroudakis G, et al. Cancers of unknown primary site: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26:v133–138.
Greco FA, Hainsworth JD. Introduction: unknown primary cancer. Semin Oncol. 2009;36:6–7.
Chang CJ, Chao CH, Xia W, Yang JY, Xiong Y, Li CW, et al. p53 regulates epithelial-mesenchymal transition and stem cell properties through modulating miRNAs. Nat Cell Biol. 2011;13:317–23.
Christiansen JJ, Rajasekaran AK. Reassessing epithelial to mesenchymal transition as a prerequisite for carcinoma invasion and metastasis. Cancer Res. 2006;66:8319–26.
Kang Y, Pantel K. Tumor cell dissemination: emerging biological insights from animal models and cancer patients. Cancer Cell. 2013;23:573–81.
Gast CE, Silk AD, Zarour L, Riegler L, Burkhart JG, Gustafson KT, et al. Cell fusion potentiates tumor heterogeneity and reveals circulating hybrid cells that correlate with stage and survival. Sci Adv. 2018;4:eaat7828.
Sutton TL, Walker BS, Wong MH. Circulating hybrid cells join the fray of circulating cellular biomarkers. Cell Mol Gastroenterol Hepatol. 2019;8:595–607.
Liu Q, Zhang R, Michalski CW, Liu B, Liao Q, Kleeff J. Surgery for synchronous and metachronous single-organ metastasis of pancreatic cancer: a SEER database analysis and systematic literature review. Sci Rep. 2020;10:4444.
Acknowledgements
We thank Atsushi Enomoto and Naoya Asai (Department of Pathology, Nagoya University Graduate School of Medicine) for providing us with the LSL-p53R172H and LSL-tdTomato mouse models.
Funding
This work was supported by the Japan Society for the Promotion of Science (JSPS) and Grants-in-Aid for Scientific Research (KAKENHI) (grant number 17K10695 and 20H03751).
Author information
Authors and Affiliations
Contributions
Conceptualization, investigation, methodology, visualization, writing, J.Y.; funding acquisition, J.Y., E.T., and T.K.; data curation and supervision, Y.Y. and T.K.; support investigation, Y.O.; and project administration, M.N.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Yamaguchi, J., Kokuryo, T., Yokoyama, Y. et al. Premalignant pancreatic cells seed stealth metastasis in distant organs in mice. Oncogene 40, 2273–2284 (2021). https://doi.org/10.1038/s41388-021-01706-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-021-01706-8
This article is cited by
-
Circulating tumor cells: biology and clinical significance
Signal Transduction and Targeted Therapy (2021)