Abstract
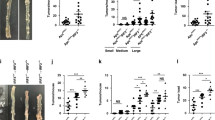
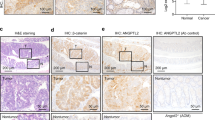
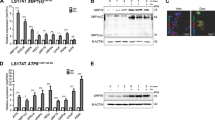
Although the Wnt/β-catenin pathway plays a central role in the carcinogenesis and maintenance of colorectal cancer (CRC), attempts to target the pathway itself have not been very successful. MyD88, an adaptor protein of the TLR/IL-1β signaling, has been implicated in the integrity of the intestines as well as in their tumorigenesis. In this study, we aimed to clarify the mechanisms by which epithelial MyD88 contributes to intestinal tumor formation and to address whether MyD88 can be a therapeutic target of CRC. Conditional knockout of MyD88 in intestinal epithelial cells (IECs) reduced tumor formation in Apc+/Δ716 mice, accompanied by decreased proliferation and enhanced apoptosis of tumor epithelial cells. Mechanistically, the MyD88 loss caused inactivation of the JNK-mTORC1, NF-κB, and Wnt/β-catenin pathways in tumor cells. Induction of MyD88 knockout in the intestinal tumor-derived organoids, but not in the normal IEC-derived organoids, induced apoptosis and reduced their growth. Treatment with the MyD88 inhibitor ST2825 also suppressed the growth of the intestinal tumor-derived organoids. Knockdown of MYD88 in human CRC cell lines with mutations in APC or CTNNB1 induced apoptosis and reduced their proliferation as well. These results indicate that MyD88 loss is synthetic lethal with mutational activation of the Wnt/β-catenin signaling in intestinal tumor epithelial cells. Inhibition of MyD88 signaling can thus be a novel therapeutic strategy for familial adenomatous polyposis (FAP) as well as for colorectal cancer harboring mutations in the Wnt/β-catenin signaling.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Fearon ER. Molecular genetics of colorectal cancer. Annu Rev Pathol. 2011;6:479–507.
Fodde R, Smits R, Clevers H. APC, signal transduction and genetic instability in colorectal cancer. Nat Rev Cancer. 2001;1:55–67.
Fearnhead NS, Britton MP, Bodmer WF. The ABC of APC. Hum Mol Genet. 2001;10:721–33.
Scholer-Dahirel A, Schlabach MR, Loo A, Bagdasarian L, Meyer R, Guo R, et al. Maintenance of adenomatous polyposis coli (APC)-mutant colorectal cancer is dependent on Wnt/β-catenin signaling. Proc Natl Acad Sci USA. 2011;108:17135–40.
Kahn M. Can we safely target the WNT pathway? Nat Rev Drug Discov. 2014;13:513–32.
Nusse R, Clevers H. Wnt/β-Catenin Signaling. Dis, Emerg Therapeutic Modalities. 2017;169:985–99.
Oshima M, Oshima H, Kitagawa K, Kobayashi M, Itakura C, Taketo M. Loss of Apc heterozygosity and abnormal tissue building in nascent intestinal polyps in mice carrying a truncated Apc gene. Proc Natl Acad Sci. 1995;92:4482–6.
Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–76.
Ngo VN, Young RM, Schmitz R, Jhavar S, Xiao W, Lim K-h, et al. Oncogenically active MYD88 mutations in human lymphoma. Nature. 2011;470:115–9.
Treon SP, Xu L, Yang G, Zhou Y, Liu X, Cao Y, et al. MYD88 L265P somatic mutation in Waldenström’s macroglobulinemia. N Engl J Med. 2012;367:826–33.
Wang EL, Qian ZR, Nakasono M, Tanahashi T, Yoshimoto K, Bando Y, et al. High expression of Toll-like receptor 4/myeloid differentiation factor 88 signals correlates with poor prognosis in colorectal cancer. Br J Cancer. 2010;102:908–15.
Rakoff-Nahoum S, Medzhitov R. Regulation of spontaneous intestinal tumorigenesis through the adaptor protein MyD88. Science. 2007;317:124–7.
Lee SH, Hu L-L, Gonzalez-Navajas J, Seo GS, Shen C, Brick J, et al. ERK activation drives intestinal tumorigenesis in Apc(min/+) mice. Nat Med. 2010;16:665–70.
Fa Scheeren, Kuo AH, van Weele LJ, Cai S, Glykofridis I, Sikandar SS, et al. A cell-intrinsic role for TLR2-MYD88 in intestinal and breast epithelia and oncogenesis. Nat Cell Biol. 2014;16:1238–48.
Fujishita T, Aoki M, Taketo MM. JNK signaling promotes intestinal tumorigenesis through activation of mTOR complex 1 in Apc(Δ716) mice. Gastroenterology. 2011;140:1556–63.e6.
Fujishita T, Aoki K, Ha Lane, Aoki M, Taketo MM. Inhibition of the mTORC1 pathway suppresses intestinal polyp formation and reduces mortality in ApcDelta716 mice. Proc Natl Acad Sci USA. 2008;105:13544–49.
Fujishita T, Aoki M, Taketo MM. The role of mTORC1 pathway in intestinal tumorigenesis. Cell cycle. 2009;8:3684–7.
Fujishita T, Kojima Y, Kajino-Sakamoto R, Taketo MM, Aoki M. Tumor microenvironment confers mTOR inhibitor resistance in invasive intestinal adenocarcinoma. Oncogene. 2017;36:6480–9.
Steinhusen U, Badock V, Bauer A, Behrens J, Wittman-Liebold B, Dörken B, et al. Apoptosis-induced cleavage of β-catenin by caspase-3 results in proteolytic fragments with reduced transactivation potential. J Biol Chem. 2000;275:16345–3.
Dhar R, Persaud SD, Mireles JR, Basu A. Proteolytic cleavage of p70 ribosomal S6 kinase by caspase-3 during DNA damage-induced apoptosis. Biochemistry. 2009;48:1474–80.
Brugarolas JB, Vazquez F, Reddy A, Sellers WR, Kaelin WG. TSC2 regulates VEGF through mTOR-dependent and -independent pathways. Cancer Cell. 2003;4:147–58.
Treins C, Giorgetti-Peraldi S, Murdaca J, Semenza GL, Van, Obberghen E. Insulin stimulates hypoxia-inducible factor 1 through a phosphatidylinositol 3-kinase/target of rapamycin-dependent signaling pathway. J Biol Chem. 2002;277:27975–81.
Zundel W, Schindler C, Haas-Kogan D, Koong A, Kaper F, Chen E, et al. Loss of PTEN facilitates HIF-1-mediated gene expression. Genes Dev. 2000;14:391–6.
Vadde R, Vemula S, Jinka R, Merchant N, Bramhachari PV, Nagaraju GP. Role of hypoxia-inducible factors (HIF) in the maintenance of stemness and malignancy of colorectal cancer. Crit Rev Oncol/Hematol. 2017;113:22–7.
Xue X, Ramakrishnan SK, Shah YM. Activation of HIF-1α does not increase intestinal tumorigenesis. Am J Physiol Gastrointest liver Physiol. 2014;307:G187–G195.
Rohwer N, Jumpertz S, Erdem M, Egners A, Warzecha KT, Fragoulis A, et al. Non-canonical HIF-1 stabilization contributes to intestinal tumorigenesis. Oncogene. 2019;38:5670–85.
Anastas JN, Moon RT. WNT signalling pathways as therapeutic targets in cancer. Nat Rev Cancer. 2013;13:11–26.
Novellasdemunt L, Antas P, Li VSW. Targeting Wnt signaling in colorectal cancer. A Review in the Theme: Cell Signaling: Proteins, Pathways and Mechanisms. Am J Physiol - Cell Physiol. 2015;309:C511–C521.
Holtorf A, Conrad A, Holzmann B, Janssen KP. Cell-type specific MyD88 signaling is required for intestinal tumor initiation and progression to malignancy. OncoImmunology. 2018;7:1–10.
Taniguchi K, Karin M. NF-B, inflammation, immunity and cancer: coming of age. Nat Rev Immunol. 2018;18:309–24.
Colomer C, Margalef P, Gonzalez J, Vert A, Bigas A, Espinosa L. IKKalpha is required in the intestinal epithelial cells for tumour stemness. Br J Cancer. 2018;118:839–46.
Schwitalla S, Fingerle AA, Cammareri P, Nebelsiek T, Göktuna SI, Ziegler PK, et al. Intestinal tumorigenesis initiated by dedifferentiation and acquisition of stem-cell-like properties. Cell. 2013;152:25–38.
Fujishita T, Kajino-Sakamoto R, Kojima Y, Taketo MM, Aoki M. Antitumor activity of the MEK inhibitor trametinib on intestinal polyp formation in Apc(Δ716) mice involves stromal COX-2. Cancer Sci. 2015;106:692–99.
Weber ANR, Cardona Gloria Y, Çınar Ö, Reinhardt HC, Pezzutto A, Wolz O-O. Oncogenic MYD88 mutations in lymphoma: novel insights and therapeutic possibilities. Cancer Immunol, Immunother. 2018;67:1797–1807.
Takaku K, Oshima M, Miyoshi H, Matsui M, Seldin MF, Taketo MM. Intestinal tumorigenesis in compound mutant mice of both Dpc4 (Smad4) and Apc genes. Cell. 1998;92:645–56.
Hou B, Reizis B, DeFranco AL. Toll-like receptors activate innate and adaptive immunity by using dendritic cell-intrinsic and -extrinsic mechanisms. Immunity. 2008;29:272–82.
Marjou FE, K-pP Janssen, BH-jJ Chang, Li M, Chan L, Louvard D, et al. Tissue-specific and inducible Cre-mediated recombination in the gut epithelium. Genesis. 2004;39:186–93.
Miyoshi H, Stappenbeck TS. In vitro expansion and genetic modification of gastrointestinal stem cells in spheroid culture. Nat Protoc. 2013;8:2471–82.
Sato T, Stange DE, Ferrante M, Vries RG, Van Es JH, Van den Brink S, et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology. 2011;141:1762–72.
Acknowledgements
We would like to thank Dr Thaddeus S. Stappenbeck and Dr Hiroyuki Miyoshi for kindly giving us L-WRN cells, Yumiko Ito for technical assistance, and Dr Ayako Demachi-Okamura for technical advice.
Funding
This work was supported by JSPS Grant-in-Aid for Scientific Research (JP18K07254) to RKS and JSPS Grant-in-Aid for Scientific Research on Innovative Areas (JP26111001) to MA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Kajino-Sakamoto, R., Fujishita, T., Taketo, M.M. et al. Synthetic lethality between MyD88 loss and mutations in Wnt/β-catenin pathway in intestinal tumor epithelial cells. Oncogene 40, 408–420 (2021). https://doi.org/10.1038/s41388-020-01541-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-020-01541-3
This article is cited by
-
Targeting MyD88: Therapeutic mechanisms and potential applications of the specific inhibitor ST2825
Inflammation Research (2023)
-
Wnt signaling in colorectal cancer: pathogenic role and therapeutic target
Molecular Cancer (2022)
-
OLFM4 deficiency delays the progression of colitis to colorectal cancer by abrogating PMN-MDSCs recruitment
Oncogene (2022)
-
Synthetic lethality in personalized cancer therapy
Genome Instability & Disease (2022)