Abstract
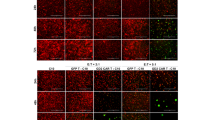
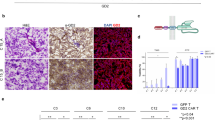
The CD56 antigen (NCAM-1) is highly expressed on several malignancies with neuronal or neuroendocrine differentiation, including small-cell lung cancer and neuroblastoma, tumor types for which new therapeutic options are needed. We hypothesized that CD56-specific chimeric antigen receptor (CAR) T cells could target and eliminate CD56-positive malignancies. Sleeping Beauty transposon-generated CD56R-CAR T cells exhibited αβT-cell receptors, released antitumor cytokines upon co-culture with CD56+ tumor targets, demonstrated a lack of fratricide, and expression of cytolytic function in the presence of CD56+ stimulation. The CD56R-CAR+ T cells are capable of killing CD56+ neuroblastoma, glioma, and SCLC tumor cells in in vitro co-cultures and when tested against CD56+ human xenograft neuroblastoma models and SCLC models, CD56R-CAR+ T cells were able to inhibit tumor growth in vivo. These results indicate that CD56-CARs merit further investigation as a potential treatment for CD56+ malignancies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23–34.
Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018–28.
Page DB, Postow MA, Callahan MK, Allison JP, Wolchok JD. Immune modulation in cancer with antibodies. Annu Rev Med. 2014;65:185–202.
Ott PA, Elez E, Hiret S, Kim DW, Morosky A, Saraf S, et al. Pembrolizumab in patients with extensive-stage small cell lung cancer: updated survival results from KEYNOTE-028 2016. J Clin Oncol. 2017;35:3823–9.
Taylor M, Antonia S, Bendell J, Calvo E, Jäger D, de Braud F, et al. Phase I/II study of nivolumab with or without ipilimumab for treatment of recurrent small cell lung cancer (SCLC): CA209-032. J Immunotherapy Cancer. 2015;3:P376.
Antonia SJ, Lopez-Martin JA, Bendell J, Ott PA, Taylor M, Eder JP, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol. 2016;17:883–95.
Yu AL, Gilman AL, Ozkaynak MF, London WB, Kreissman SG, Chen HX, et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med. 2010;363:1324–34.
Chen HL, Gabrilovich D, Tampe R, Girgis KR, Nadaf S, Carbone DP. A functionally defective allele of TAP1 results in loss of MHC class I antigen presentation in a human lung cancer. Nat Genet. 1996;13:210–3.
Zaretsky JM, Garcia-Diaz A, Shin DS, Escuin-Ordinas H, Hugo W, Hu-Lieskovan S, et al. Mutations associated with acquired resistance to PD-1 blockade in melanoma. N Engl J Med. 2016;375:819–29.
Yoshihama S, Roszik J, Downs I, Meissner TB, Vijayan S, Chapuy B, et al. NLRC5/MHC class I transactivator is a target for immune evasion in cancer. Proc Natl Acad Sci USA. 2016;113:5999–6004.
Dalmau J, Graus F, Cheung NK, Rosenblum MK, Ho A, Canete A, et al. Major histocompatibility proteins, anti-Hu antibodies, and paraneoplastic encephalomyelitis in neuroblastoma and small cell lung cancer. Cancer. 1995;75:99–109.
Jena B, Dotti G, Cooper LJ. Redirecting T-cell specificity by introducing a tumor-specific chimeric antigen receptor. Blood. 2010;116:1035–44.
Shah MH, Lorigan P, O’Brien ME, Fossella FV, Moore KN, Bhatia S, et al. Phase I study of IMGN901, a CD56-targeting antibody-drug conjugate, in patients with CD56-positive solid tumors. Invest New Drugs. 2016;34:290–9.
Feng Y, Wang Y, Zhu Z, Li W, Sussman RT, Randall M, et al. Differential killing of CD56-expressing cells by drug-conjugated human antibodies targeting membrane-distal and membrane-proximal non-overlapping epitopes. MAbs. 2016;8:799–810.
Graus F, Dalmou J, Rene R, Tora M, Malats N, Verschuuren JJ, et al. Anti-Hu antibodies in patients with small-cell lung cancer: association with complete response to therapy and improved survival. J Clin Oncol. 1997;15:2866–72.
Kazarian M, Laird-Offringa IA. Small-cell lung cancer-associated autoantibodies: potential applications to cancer diagnosis, early detection, and therapy. Mol Cancer. 2011;10:33.
Winter SF, Sekido Y, Minna JD, McIntire D, Johnson BE, Gazdar AF, et al. Antibodies against autologous tumor cell proteins in patients with small-cell lung cancer: association with improved survival. J Natl Cancer Inst. 1993;85:2012–8.
Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–70.
Noble A, Giorgini A, Leggat JA. Cytokine-induced IL-10-secreting CD8 T cells represent a phenotypically distinct suppressor T-cell lineage. Blood. 2006;107:4475–83.
Kelly-Rogers J, Madrigal-Estebas L, O’Connor T, Doherty DG. Activation-induced expression of CD56 by T cells is associated with a reprogramming of cytolytic activity and cytokine secretion profile in vitro. Hum Immunol 2006;67:863–73.
Ohkawa T, Seki S, Dobashi H, Koike Y, Habu Y, Ami K, et al. Systematic characterization of human CD8 + T cells with natural killer cell markers in comparison with natural killer cells and normal CD8 + T cells. Immunology. 2001;103:281–90.
Pittet MJ, Speiser DE, Valmori D, Cerottini JC, Romero P. Cutting edge: cytolytic effector function in human circulating CD8 + T cells closely correlates with CD56 surface expression. J Immunol. 2000;164:1148–52.
Socinski MA, Kaye FJ, Spigel DR, Kudrik FJ, Ponce S, Ellis PM, et al. Phase 1/2 study of the CD56-targeting antibody-drug conjugate lorvotuzumab mertansine (IMGN901) in combination with carboplatin/etoposide in small-cell lung cancer patients with extensive-stage disease. Clin Lung Cancer. 2017;18:68–76. e62
Whiteman KR, Johnson HA, Mayo MF, Audette CA, Carrigan CN, LaBelle A, et al. Lorvotuzumab mertansine, a CD56-targeting antibody-drug conjugate with potent antitumor activity against small cell lung cancer in human xenograft models. MAbs. 2014;6:556–66.
Doyle A, Martin WJ, Funa K, Gazdar A, Carney D, Martin SE, et al. Markedly decreased expression of class I histocompatibility antigens, protein, and mRNA in human small-cell lung cancer. J Exp Med. 1985;161:1135–51.
Fisk B, Ioannides CG, Aggarwal S, Wharton JT, O’Brian CA, Restifo N, et al. Enhanced expression of HLA-A,B,C and inducibility of TAP-1, TAP-2, and HLA-A,B,C by interferon-gamma in a multidrug-resistant small cell lung cancer line. Lymphokine Cytokine Res. 1994;13:125–31.
Yazawa T, Ito T, Kamma H, Suzuki T, Okudela K, Hayashi H, et al. Complicated mechanisms of class II transactivator transcription deficiency in small cell lung cancer and neuroblastoma. Am J Pathol. 2002;161:291–300.
Ryan SO, Turner MS, Gariepy J, Finn OJ. Tumor antigen epitopes interpreted by the immune system as self or abnormal-self differentially affect cancer vaccine responses. Cancer Res. 2010;70:5788–96.
Bruns M, Wanger J, Schumacher U, Deppert W. T-cell epitope strength in WAP-T mouse mammary carcinomas is an important determinant in PD1/PD-L1 immune checkpoint blockade therapy. Oncotarget. 2016;7:64543–59.
Michielsen AJ, Noonan S, Martin P, Tosetto M, Marry J, Biniecka M, et al. Inhibition of dendritic cell maturation by the tumor microenvironment correlates with the survival of colorectal cancer patients following bevacizumab treatment. Mol Cancer Ther. 2012;11:1829–37.
Sakemura R, Terakura S, Watanabe K, Julamanee J, Takagi E, Miyao K, et al. A Tet-on inducible system for controlling CD19-chimeric antigen receptor expression upon drug administration. Cancer Immunol Res. 2016;4:658–68.
Roellecke K, Virts EL, Einholz R, Edson KZ, Altvater B, Rossig C, et al. Optimized human CYP4B1 in combination with the alkylator prodrug 4-ipomeanol serves as a novel suicide gene system for adoptive T-cell therapies. Gene Ther. 2016;23:615–26.
Fedorov VD, Themeli M, Sadelain M. PD-1- and CTLA-4-based inhibitory chimeric antigen receptors (iCARs) divert off-target immunotherapy responses. Sci Transl Med. 2013;5:215ra172.
Morsut L, Roybal KT, Xiong X, Gordley RM, Coyle SM, Thomson M, et al. Engineering customized. Cell Sens Response Behav Using Synth Notch Recept Cell. 2016;164:780–91.
Singh H, Manuri PR, Olivares S, Dara N, Dawson MJ, Huls H, et al. Redirecting specificity of T-cell populations for CD19 using the Sleeping Beauty system. Cancer Res. 2008;68:2961–71.
Singh H, Figliola MJ, Dawson MJ, Huls H, Olivares S, Switzer K, et al. Reprogramming CD19-specific T cells with IL-21 signaling can improve adoptive immunotherapy of B-lineage malignancies. Cancer Res. 2011;71:3516–27.
Acknowledgements
We thank the flow cytometry and cellular imaging core facilities at MD Anderson. This study was funded by Cancer Center Core Grant (CA16672); RO1 (CA124782, CA120956, CA141303, CA141303); P01 (CA148600); SPORES (CA100632, CA136411, CA00632); Albert J Ward Foundation; Alex’s Lemonade Stand Foundation; Burroughs Wellcome Fund; Cancer Prevention and Research Institute of Texas; Charles B. Goddard Foundation of Texas; CLL Global Research Foundation; Energy Transfer Partners; Estate of Noelan L. Bibler; Gillson Longenbaugh Foundation; Harry T. Mangurian, Jr., Fund for Leukemia Immunotherapy; Khalifa Bin Zayed Al Nahyan Foundation; Kleberg Foundation; Leukemia and Lymphoma Society; Lung Cancer Research Foundation; Lung Cancer Moon Shot; Lymphoma Research Foundation; Miller Foundation; Mr. Herb Simons; Mr. and Mrs. Joe H. Scales; Mr. Thomas Scott; National Foundation for Cancer Research; NIH Cancer Center Support Grant (CA016672) Pediatric Cancer Research Foundation; Sheikh Khalifa Bin Zayed Al Nahyan Institute for Personalized Cancer Therapy; University of Texas MD Anderson Cancer Center Sister Institution Network Fund and Moon Shot Fund; The University of Texas Southwestern Medical Center and The University of Texas MD Anderson Cancer Center NIH Lung SPORE Grant (5 P50 CA070907); William Lawrence and Blanche Hughes Children’s Foundation. Publication under the Creative Commons CC-BY license is not required.
Authors contributions
DLC and WLD designed and conducted experiments, performed data interpretation, and manuscript preparation; MHH provided manufacture assistance with the SB plasmids and CAR T cells; HS and SO assisted with the molecular work; TM and KS assisted with the animal experiments; LJC, KAD, BSG, and JVH provided concept and design of work and supervision of scientific work. LJC and JVH provided manuscript editing and review of this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The technology described in this report was advanced through research conducted at MD Anderson by LJC. In January 2015, the technology was licensed by MD Anderson for commercial application to ZIOPHARM Oncology, Inc., and Intrexon Corporation, in exchange for equity interests in each of these companies. LJC and some co-authors are eligible to receive equity as a result of the licensing of this technology. On May 7, 2015, LJC was appointed as the Chief Executive Officer at ZIOPHARM. LJC is also a Visiting Scientist at MD Anderson, where he continues to help supervise the development of this technology. The information being reported in this publication is research in which MD Anderson has an institutional financial conflict of interest. Because MD Anderson is committed to the protection of human subjects and the effective management of its financial conflicts of interest in relation to its research activities, MD Anderson has implemented an Institutional Conflict of Interest Management and Monitoring Plan to manage and monitor the conflict of interest with respect to MD Anderson’ s conduct of this research. JVH reports receiving commercial research support from AstraZeneca and is a consultant/advisory board member for AstraZeneca, Boehringer Ingelheim, Genentech, GlaxoSmithKline, and Synta. The remaining authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Crossland, D.L., Denning, W.L., Ang, S. et al. Antitumor activity of CD56-chimeric antigen receptor T cells in neuroblastoma and SCLC models. Oncogene 37, 3686–3697 (2018). https://doi.org/10.1038/s41388-018-0187-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-018-0187-2
This article is cited by
-
Orchestrating smart therapeutics to achieve optimal treatment in small cell lung cancer: recent progress and future directions
Journal of Translational Medicine (2023)
-
Novel Therapeutic Options for Small Cell Lung Cancer
Current Oncology Reports (2023)
-
Signal pathways and precision therapy of small-cell lung cancer
Signal Transduction and Targeted Therapy (2022)
-
Infiltrating T lymphocytes in the tumor microenvironment of small cell lung cancer: a state of knowledge review
Journal of Cancer Research and Clinical Oncology (2022)
-
Clinicopathological study of hepatic mesenchymal hamartoma and undifferentiated embryonal sarcoma of the liver: a single center study from Iran
Diagnostic Pathology (2021)