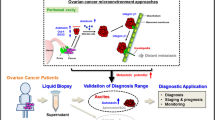
Abstract
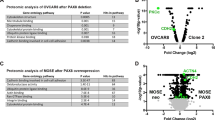
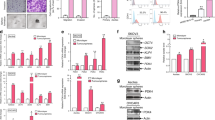
Epithelial ovarian carcinoma (EOC) patients often acquire resistance against common chemotherapeutic drugs like paclitaxel and cisplatin. The mechanism responsible for the same is ambiguous. We have identified a putative drug-resistant tumour cell phenotype (EpCAM+CD45+) in the ascitic fluid of EOC patients, which appears to originate from the primary tumour. These cells represent the major tumour burden and are more drug resistant compared to EpCAM+ tumour cells due to the over-expression of SIRT1, ABCA1 and BCL2 genes. We have found that the entire EpCAM+CD45+ population is highly invasive with signature mesenchymal gene expression and also consists of subpopulations of ovarian cancer stem cells (CD133+ and CD117+CD44+). Additionally, we demonstrate that the EpCAM+CD45+ tumour cells over-express major histocompatibility complex class I antigen, which enable them to evade the natural killer cell-mediated immune surveillance. Preliminary evidence obtained in OVCAR-5 cells suggests that exosomes, secreted by non-tumour cells of the ascitic fluid, play an important role in rendering drug resistance and invasive properties to the cancer cells. Identification of such aggressive tumour cells and deciphering their origin is important for designing better drug targets for EOC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Paley PJ. Ovarian cancer screening: are we making any progress? Curr Opin Oncol. 2001;13:399–402.
Ozols RF. Systemic therapy for ovarian cancer: current status and new treatments. Semin Oncol. 2006;33(Suppl 6):S3–11.
Wang Z, Li Y, Kong D, Banerjee S, Ahmed A, Azmi AS, et al. Acquisition of epithelial–mesenchymal transition phenotype of gemcitabine-resistant pancreatic cancer cells is linked with activation of the notch signaling pathway. Cancer Res. 2009;69:2400–2407.
Ahmed N, Abubaker K, Findlay J, Quinn M. Epithelial mesenchymal transition and cancer stem cell-like phenotypes facilitate chemo resistance in recurrent ovarian cancer. Curr Cancer Drug Target. 2010;10:268–178.
Mani SA, Guo W, Liao M-J, Eaton EN, Ayyanan A, Zhou AY, et al. The epithelial–mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–15.
Kim Y, Joo KM, Jin J, Nam D-H. Cancer stem cells and their mechanisms of chemo-radiation resistance. Int J Stem Cells. 2009;2:109–14.
Creighton CJ, Li X, Landis M, Dixon JM, Neumeister VM, Sjolund A, et al. Residual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating features. Proc Natl Acad Sci USA. 2009;106:13820–5.
Chang L, Graham PH, Hao J, Ni J, Bucci J, Cozzi PJ, et al. Acquisition of epithelial–mesenchymal transition and cancer stem cell phenotypes is associated with activation of the PI3K/Akt/mTOR pathway in prostate cancer radio resistance. Cell Death Dis. 2013;4:e875.
Li H, Zeng J, Shen K. PI3K/AKT/mTOR signaling pathway as a therapeutic target for ovarian cancer. Arch Gynecol Obstet. 2014;290:1067–78.
Sunayama J, Matsuda K, Sato A, Tachibana K, Suzuki K, Narita Y, et al. Crosstalk between the PI3K/mTOR and MEK/ERK pathways involved in the maintenance of self-renewal and tumorigenicity of glioblastoma stem-like cells. Stem Cells. 2010;28:1930–9.
Shain KH, Dalton WS, Tao J. The tumor microenvironment shapes hallmarks of mature B-cell malignancies. Oncogene. 2015;34:4673–82.
Olumi AF, Grossfeld GD, Hayward SW, Carroll PR, Tlsty TD, Cunha GR. Carcinoma-associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer Res. 1999;59:5002–11.
Quail DF, Joyce JA, Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423–37.
Barker N, Clevers H. Tumor environment: a potent driving force in colorectal cancer? Trends Mol Med. 2001;7:535–7.
Olumi AF, Grossfeld GD, Hayward SW, Carroll PR, Tlsty TD, Cunha GR. Carcinoma-associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer Res. 1999;59:5002–11.
Schwitalla S, Fingerle AA, Cammareri P, Nebelsiek T, Goktuna SI, Ziegler PK, et al. Intestinal tumorigenesis initiated by dedifferentiation and acquisition of stem-cell-like properties. Cell. 2013;152:25–38.
Powell AE, Anderson EC, Davies PS, Silk AD, Pelz C, Impey S, et al. Fusion between intestinal epithelial cells and macrophages in a cancer context results in nuclear reprogramming. Cancer Res. 2011;71:1497–505.
Ramakrishnan M, Mathur SR, Mukhopadhyay A. Fusion-derived epithelial cancer cells express hematopoietic markers and contribute to stem cell and migratory phenotype in ovarian carcinoma. Cancer Res. 2013;73:5360–70.
Luga V, Zhang L, Viloria-Petit AM, Ogunjimi AA, Inanlou MR, Chiu E, et al. Exosomes mediate stromal mobilization of autocrine Wnt-PCP signaling in breast cancer cell migration. Cell. 2012;151:1542–56.
RoccaroAM SaccoA, MaisoP AzabAK, Tai Y-T, Reagan M, et al. BM mesenchymal stromal cell-derived exosomes facilitate multiple myeloma progression. J Clin Invest. 2013;123:1542–55.
Ono ZM, Kosaka N, Tominaga N, Yoshioka Y, Takeshita F, Takahashi RU, et al. Exosomes from bone marrow mesenchymal stem cells contain a microRNA that promotes dormancy in metastatic breast cancer cells. Sci Signal. 2014;7:ra63.
Rubin SC, Randall TC, Armstrong KA, Chi DS, Hoskins WJ. Ten-year follow-up of ovarian cancer patients after second-look laparotomy with negative findings. Obstet Gynecol. 1999;93:21–24.
Zhang S, Balch C, Chan MW, Lai H-C, Matei D, Schilder JM, et al. Identification and characterization of ovarian cancer-initiating cells from primary human tumors. Cancer Res. 2008;68:4311–20.
Baba T, Convery PA, Matsumura N, Whitaker RS, Kondoh E, Perry T, et al. Epigenetic regulation of CD133 and tumorigenicity of CD133+ ovarian cancer cells. Oncogene. 2009;28:209–18.
Silva IA, Bai S, McLean K, Yang K, Griffith K, Thomas D, et al. Aldehyde dehydrogenase in combination with CD133 defines angiogenic ovarian cancer stem cells that portend poor patient survival. Cancer Res. 2011;71:3991–4001.
Bapat SA, Mali AM, Koppikar CB, Kurrey N. Stem and progenitor-like cells contribute to the aggressive behaviour of human epithelial ovarian cancer. Cancer Res. 2005;65:3025–9.
Craveiro V, Yang-Hartwich Y, Holmberg JC, Sumi NJ, Pizzonia J, Griffin B, et al. Phenotypic modifications in ovarian cancer stem cells following Paclitaxel treatment. Cancer Med. 2013;2:751–62.
Houghton J, Stoicov C, Nomura S, Rogers AB, Carlson J, Li H, et al. Gastric cancer originating from bone marrow-derived cells. Science. 2004;306:1568–71.
Liu C, Chen Z, Chen Z, Zhang T, Lu Y. Multiple tumor types may originate from bone marrow-derived cells. Neoplasia. 2006;8:716–24.
Avital I, Moreira AL, Klimstra DS, Leversha M, Papadopoulos EB, Brennan M, et al. Donor-derived human bone marrow cells contribute to solid organ cancers developing after bone marrow transplantation. Stem Cells. 2007;25:2903–9.
Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–44.
Larizza L, Schirrmacher V, Pfluger E. Acquisition of high metastatic capacity after in vitro fusion of a non-metastatic tumor line with a bone marrow-derived macrophage. J Exp Med. 1984;160:1579–84.
Rizv AZ, Swain JR, Davies PS, Bailey AS, Decker AD, Willenbring H, et al. Bone marrow-derived cells fuse with normal and transformed intestinal stem cells. Proc Natl Acad Sci USA. 2006;103:6321–5.
Al-Ejeh F, Simpson PT, Sanus JM, Klein K, Kalimutho M, Shi W, et al. Meta-analysis of the global gene expression profile of triple-negative breast cancer identifies genes for the prognostication and treatment of aggressive breast cancer. Oncogenesis. 2014;3:e100.
Latifi A, Luwor RB, Bilandzic M, Nazaretian S, Stenvers K, Pyman J, et al. Isolation and characterization of tumor cells from the ascites of ovarian cancer patients: Molecular phenotype of chemoresistant ovarian tumors. PLoS ONE. 2012;7:e46858.
Wicha MS, Liu S, Dontu G. Cancer stem cells: an old idea—a paradigm shift. Cancer Res. 2006;66:1883–90.
Steg AD, Bevis KS, Katre AA, Ziebarth A, Dobbin ZC, Alvarez RD, et al. Stem cell pathways contribute to clinical chemoresistance in ovarian cancer. Clin Cancer Res. 2012;18:869–81.
Ahmed N, Abubaker K, Findlay J, Quinn M. Cancerous ovarian stem cells: obscure targets for therapy but relevant to chemoresistance. J Cell Biochem. 2013;114:21–34.
Latifi A, Abubaker K, Castrechini N, Ward AC, Liongue C, Dobill F, et al. Cisplatin treatment of primary and metastatic epithelial ovarian carcinomas generates residual cells with mesenchymal stem cell-like profile. J Cell Biochem. 2011;112:2850–64.
Liu M, Casimiro MC, Wang C, Shirley LA, Jiao X, Katiyar S, et al. p21CIP1 attenuates Ras- and c-Myc-dependent breast tumor epithelial mesenchymal transition and cancer stem cell-like gene expression in vivo. Proc Natl Acad Sci USA. 2009;106:19035–9.
Matte I, Lane D, Laplante C, Piché A. Profiling of cytokines in human epithelial ovarian cancer ascites. Am J Cancer Res. 2012;2:566–80.
Smyth MJ, Hayakawa Y, Takeda K, Yagita H. New aspects of natural-killer-cell surveillance and therapy of cancer. Nat Rev. 2002;2:850–86.
Bamias A, Tsiatas ML, Kafantari E, Liakou C, Rodolakis A, Voulgaris Z, et al. Significant differences of lymphocytes isolated from ascites of patients with ovarian cancer compared to blood and tumor lymphocytes: Association of CD3+CD56+ cells with platinum resistance. Gynecol Oncol. 2007;106:75–81.
Gubbels JAA, Felder M, Horibata S, Belisle JA, Kapur A, Holden H, et al. MUC16 provides immune protection by inhibiting synapse formation between NK and ovarian tumor cells. Mol Cancer. 2010;9:11.
Rousalova I, Krepela E. Granzyme B-induced apoptosis in cancer cells and its regulation. Int J Oncol. 2010;37:1361–78.
Wang RF. The role of MHC class II-restricted tumor antigens and CD4+ T cells in antitumor immunity. Trends Immunol. 2001;22:269–76.
Ahmed N, Stenvers KL. Getting to know ovarian cancer ascites: opportunities for targeted therapy-based translational research. Front Oncol. 2013;3:256.
Lee YI, Andaloussi SEL, Wood MJA. Exosomes and microvesicles: extracellular vesicles for genetic information transfer and gene therapy. Hum Mol Genet. 2012;21:R125–R134.
Runz S, Keller S, Rupp C, Stoeck A, Issa Y, Koensgen D, et al. Malignant ascites-derived exosomes of ovarian carcinoma patients contain CD24 and EpCAM. Gynecol Oncol. 2007;107:563–71.
Taylor DD, Gercel-Taylor C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol Oncol. 2008;110:13–21.
Thery C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol 2006. https://doi.org/10.1002/0471143030.cb0322s30.
Witwer KW, Buzás EI, Bemis LT, Bora A, Lässer C, Lötvall J, et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J Extracell Vesicles 2013; 2. https://doi.org/10.3402/jev.v2i0.20360.
Baligar P, Mukherjee S, Kochat V, Rastogi A, Mukhopadhyay A. Molecular and cellular functions distinguish superior therapeutic efficiency of bone marrow CD45 cells over mesenchymal stem cells in liver cirrhosis. Stem Cells. 2016;34:135–47.
Qian J, Bai H, Gao Z, Dong YU, Pei J, Ma M, et al. Downregulation of HIF-1α inhibits the proliferation and invasion of non-small cell lung cancer NCI-H157 cells. Oncol Lett. 2016;11:1738–44.
Baligar P, Kochat V, Equbal Z, Arindkar SK, Mukherjee S, Patel S, et al. Bone marrow stem cell therapy partially ameliorates pathological consequences of liver in mice expressing mutant human α1-antitrypsin. Hepatology. 2017;65:1219–335.
Acknowledgements
AM is grateful for the generous support provided by the Department of Biotechnology, Government of India to the Centre of Molecular Medicine, NII. We would like to thank Mr. Nila Ram and Mr. Ajay Sharma for acquiring confocal images. AM would like to thank Dr. Ayub Qadri, Scientist (NII, New Delhi), Prof. NK Mehra (AIIMS, New Delhi) and Dr. Awadhesh Pandit (NCBS, Bangalore) for valuable discussion in some parts of the study. Also, we are thankful to Dr. TR Santhosh Kumar (RGCB, Thiruvananthapuram, Kerala) for providing the OVCAR-5 cell line.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Akhter, M.Z., Sharawat, S.K., Kumar, V. et al. Aggressive serous epithelial ovarian cancer is potentially propagated by EpCAM+CD45+ phenotype. Oncogene 37, 2089–2103 (2018). https://doi.org/10.1038/s41388-017-0106-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-017-0106-y
This article is cited by
-
Circulating tumor cells shielded with extracellular vesicle-derived CD45 evade T cell attack to enable metastasis
Signal Transduction and Targeted Therapy (2024)
-
NK cells are never alone: crosstalk and communication in tumour microenvironments
Molecular Cancer (2023)
-
Cross-talk between cancer stem cells and immune cells: potential therapeutic targets in the tumor immune microenvironment
Molecular Cancer (2023)
-
Histone modifications in drug-resistant cancers: From a cancer stem cell and immune evasion perspective
Experimental & Molecular Medicine (2023)
-
New inflammatory indicators for cell-based liquid biopsy: association of the circulating CD44+/CD24− non-hematopoietic rare cell phenotype with breast cancer residual disease
Journal of Cancer Research and Clinical Oncology (2023)