Abstract
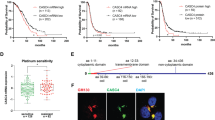
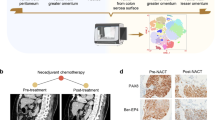
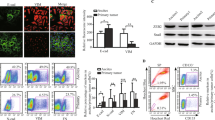
High grade serous ovarian cancer (HGSOC) is the fifth leading cause of cancer deaths among women yet effective targeted therapies against this disease are limited. The heterogeneity of HGSOC, including few shared oncogenic drivers and origination from both the fallopian tube epithelium (FTE) and ovarian surface epithelium (OSE), has hampered development of targeted drug therapies. PAX8 is a lineage-specific transcription factor expressed in the FTE that is also ubiquitously expressed in HGSOC where it is an important driver of proliferation, migration, and cell survival. PAX8 is not normally expressed in the OSE, but it is turned on after malignant transformation. In this study, we use proteomic and transcriptomic analysis to examine the role of PAX8 leading to increased migratory capabilities in a human ovarian cancer model, as well as in tumor models derived from the OSE and FTE. We find that PAX8 is a master regulator of migration with unique downstream transcriptional targets that are dependent on the cell’s site of origin. Importantly, we show that targeting PAX8, either through CRISPR genomic alteration or through drug treatment with micelle encapsulated thiostrepton, leads to a reduction in tumor burden. These findings suggest PAX8 is a unifying protein driving metastasis in ovarian tumors that could be developed as an effective drug target to treat HGSOC derived from both the OSE and FTE.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30.
Ozcan A, Shen SS, Hamilton C, Anjana K, Coffey D, Krishnan B, et al. PAX 8 expression in non-neoplastic tissues, primary tumors, and metastatic tumors: a comprehensive immunohistochemical study. Mod Pathol. 2011;24:751–64.
McCloskey CW, Goldberg RL, Carter LE, Gamwell LF, Al-Hujaily EM, Collins O, et al. A new spontaneously transformed syngeneic model of high-grade serous ovarian cancer with a tumor-initiating cell population. Front Oncol. 2014;4:53.
Tanwar PS, Mohapatra G, Chiang S, Engler DA, Zhang L, Kaneko-Tarui T, et al. Loss of LKB1 and PTEN tumor suppressor genes in the ovarian surface epithelium induces papillary serous ovarian cancer. Carcinogenesis. 2014;35:546–53.
Rodgers LH, Ó hAinmhire E, Young AN, Burdette JE. Loss of PAX8 in high-grade serous ovarian cancer reduces cell survival despite unique modes of action in the fallopian tube and ovarian surface epithelium. Oncotarget. 2016;7:32785–95.
Di Palma T, Lucci V, de Cristofaro T, Filippone MG, Zannini M. A role for PAX8 in the tumorigenic phenotype of ovarian cancer cells. Bmc Cancer. 2014;14:292.
Piek JM, van Diest PJ, Zweemer RP, Jansen JW, Poort-Keesom RJ, Menko FH, et al. Dysplastic changes in prophylactically removed Fallopian tubes of women predisposed to developing ovarian cancer. J Pathol. 2001;195:451–6.
Labidi-Galy SI, Papp E, Hallberg D, Niknafs N, Adleff V, Noe M, et al. High grade serous ovarian carcinomas originate in the fallopian tube. Nat Commun. 2017;8:1093.
Kurman RJ, Shih I-M. Molecular pathogenesis and extraovarian origin of epithelial ovarian cancer–shifting the paradigm. Hum Pathol. 2011;42:918–31.
Elias KM, Emori MM, Westerling T, Long H, Budina-Kolomets A, Li F, et al. Epigenetic remodeling regulates transcriptional changes between ovarian cancer and benign precursors. JCI Insight. 2016;1. https://doi.org/10.1172/jci.insight.87988.
Adler EK, Corona RI, Lee JM, Rodriguez-Malave N, Mhawech-Fauceglia P, Sowter H, et al. The PAX8 cistrome in epithelial ovarian cancer. Oncotarget. 2017;8:108316–32.
Mansouri A, Chowdhury K, Gruss P. Follicular cells of the thyroid gland require Pax8 gene function. Nat Genet. 1998;19:87–90.
Li CG, Nyman JE, Braithwaite AW, Eccles MR. PAX8 promotes tumor cell growth by transcriptionally regulating E2F1 and stabilizing RB protein. Oncogene. 2011;30:4824–34.
Ghannam-Shahbari D, Jacob E, Kakun RR, Wasserman T, Korsensky L, Sternfeld O, et al. PAX8 activates a p53-p21-dependent pro-proliferative effect in high grade serous ovarian carcinoma. Oncogene. 2018;37:2213.
Haley J, Tomar S, Pulliam N, Xiong S, Perkins SM, Karpf AR, et al. Functional characterization of a panel of high-grade serous ovarian cancer cell lines as representative experimental models of the disease. Oncotarget. 2016;7:32810–20.
King SM, Quartuccio SM, Vanderhyden BC, Burdette JE. Early transformative changes in normal ovarian surface epithelium induced by oxidative stress require akt upregulation, DNA damage, and epithelial-stromal interaction. Carcinogenesis. 2013;34:1125–33.
Eddie SL, Quartuccio SM, Ó hAinmhir E, Moyle-Heyrman G, Lantvit DD, Wei J-J, et al. Tumorigenesis and peritoneal colonization from fallopian tube epithelium. Oncotarget. 2015;6:20500–12.
Russo A, Czarnecki AA, Dean M, Modi DA, Lantvit DD, Hardy L, et al. PTEN loss in the fallopian tube induces hyperplasia and ovarian tumor formation. Oncogene. 2018;37:1976.
Ran FA, et al. Genome engineering using the CRISPR-Cas9 system. Nat Protoc. 2013;8:2281–308.
Endsley MP, Moyle-Heyrman G, Karthikeyan S, Lantvit DD, Davis DA, Wei J-J, et al. Spontaneous transformation of murine oviductal epithelial cells: a model system to investigate the onset of fallopian-derived tumors. Front Oncol. 2015;5:154.
Wiśniewski JR, Zougman A, Nagaraj N, Mann M. Universal sample preparation method for proteome analysis. Nat Methods. 2009;6:359–62.
Batth TS, Francavilla C, Olsen JV. Off-line high-ph reversed-phase fractionation for in-depth phosphoproteomics. J Proteome Res. 2014;13:6176–86.
Gokce E, Andrews GL, Dean RA, Muddiman DC. Increasing proteome coverage with offline RP HPLC coupled to online RP nanoLC-MS. J Chromatogr B Anal Technol Biomed Life Sci. 2011;879:610–4.
Adusumilli R, Mallick P. Data conversion with ProteoWizard msConvert. Methods Mol Biol Clifton NJ. 2017;1550:339–68.
Ong S-E, Mann M. A practical recipe for stable isotope labeling by amino acids in cell culture (SILAC). Nat Protoc. 2006;1:2650–60.
King SM, et al. The impact of ovulation on fallopian tube epithelial cells: evaluating three hypotheses connecting ovulation and serous ovarian cancer. Endocr Relat Cancer. 2011;18:627–42.
Martiny-Baron G, Kazanietz MG, Mischak H, Blumberg PM, Kochs G, Hug H, et al. Selective inhibition of protein kinase C isozymes by the indolocarbazole Gö 6976. J Biol Chem. 1993;268:9194–7.
Ke N, Wang X, Xu X, Abassi YA. The xCELLigence system for real-time and label-free monitoring of cell viability. In: Mammalian cell viability. Springer; 2011, pp 33–43.
Esparza K, Onyuksel H. Development of co-solvent freeze-drying method for the encapsulation of water-insoluble thiostrepton in sterically stabilized micelles. Int J Pharm. 2019;556:21–29.
de Cristofaro T, Di Palma T, Soriano AA, Monticelli A, Affinito O, Cocozza S, et al. Candidate genes and pathways downstream of PAX8 involved in ovarian high-grade serous carcinoma. Oncotarget. 2016;7:41929–47.
Hegde NS, Sanders DA, Rodriguez R, Balasubramanian S. The transcription factor FOXM1 is a cellular target of the natural product thiostrepton. Nat Chem. 2011;3:725–31.
Wang M, Gartel AL. Micelle-encapsulated thiostrepton as an effective nanomedicine for inhibiting tumor growth and for suppressing FOXM1 in human xenografts. Mol Cancer Ther. 2011;10:2287–97.
Jiang L, Wu X, Wang P, Wen T, Yu C, Wei L, et al. Targeting FoxM1 by thiostrepton inhibits growth and induces apoptosis of laryngeal squamous cell carcinoma. J Cancer Res Clin Oncol. 2015;141:971–81.
Ashok B, Arleth L, Hjelm RP, Rubinstein I, Onyüksel H. In vitro characterization of PEGylated phospholipid micelles for improved drug solubilization: effects of PEG chain length and PC incorporation. J Pharm Sci. 2004;93:2476–87.
Lim SB, Banerjee A, Önyüksel H. Improvement of drug safety by the use of lipid-based nanocarriers. J Control Release J Control Release Soc. 2012;163:34–45.
Laury AR, Hornick JL, Perets R, Krane JF, Corson J, Drapkin R, et al. PAX8 reliably distinguishes ovarian serous tumors from malignant mesothelioma. Am J Surg Pathol. 2010;34:627–35.
Cheung HW, Cowley GS, Weir BA, Boehm JS, Rusin S, Scott JA, et al. Systematic investigation of genetic vulnerabilities across cancer cell lines reveals lineage-specific dependencies in ovarian cancer. Proc Natl Acad Sci USA. 2011;108:12372–7.
Musrap N, Tuccitto A, Karagiannis GS, Saraon P, Batruch I, Diamandis EP. Comparative proteomics of ovarian cancer aggregate formation reveals an increased expression of calcium-activated chloride channel regulator 1 (CLCA1). J Biol Chem 2015;290:17218–27.
Grassi ML, Palma C, de S, Thomé CH, Lanfredi GP, Poersch A, Faça VM. Proteomic analysis of ovarian cancer cells during epithelial-mesenchymal transition (EMT) induced by epidermal growth factor (EGF) reveals mechanisms of cell cycle control. J Proteom. 2017;151:2–11.
Waldemarson S, Krogh M, Alaiya A, Kirik U, Schedvins K, Auer G, et al. Protein expression changes in ovarian cancer during the transition from benign to malignant. J Proteome Res. 2012;11:2876–89.
Wang L-N, Tong S-W, Hu H-D, Ye F, Li S-L, Ren H, et al. Quantitative proteome analysis of ovarian cancer tissues using a iTRAQ approach. J Cell Biochem. 2012;113:3762–72.
Harms JM, Wilson DN, Schluenzen F, Connell SR, Stachelhaus T, Zaborowska Z, et al. Translational regulation via L11: molecular switches on the ribosome turned on and off by thiostrepton and micrococcin. Mol Cell. 2008;30:26–38.
Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474: 609–15.
Zhang X, Cheng L, Minn K, Madan R, Godwin AK, Shridhar V, et al. Targeting of mutant p53-induced FoxM1 with thiostrepton induces cytotoxicity and enhances carboplatin sensitivity in cancer cells. Oncotarget. 2014;5:11365–80.
Cristofaro T, de, Mascia A, Pappalardo A, D’Andrea B, Nitsch L, Zannini M. Pax8 protein stability is controlled by sumoylation. J Mol Endocrinol. 2009;42:35–46.
Acknowledgements
This work was supported by the Department of Defense Ovarian Cancer Fund 160076, the National Cancer Institute F30CA224986, the National Cancer Center for Research Resources Facilities Improvement Program C06RR15482, the University of Illinois-Chicago Department of Chemistry, the Ara Parsegian Medical Research Foundation, and the Abraham Lincoln Fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Hardy, L.R., Pergande, M.R., Esparza, K. et al. Proteomic analysis reveals a role for PAX8 in peritoneal colonization of high grade serous ovarian cancer that can be targeted with micelle encapsulated thiostrepton. Oncogene 38, 6003–6016 (2019). https://doi.org/10.1038/s41388-019-0842-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-019-0842-2
This article is cited by
-
The renin-angiotensin-aldosterone system (RAAS) signaling pathways and cancer: foes versus allies
Cancer Cell International (2023)
-
Amplified therapeutic targets in high-grade serous ovarian carcinoma – a review of the literature with quantitative appraisal
Cancer Gene Therapy (2023)
-
Thiostrepton induces ferroptosis in pancreatic cancer cells through STAT3/GPX4 signalling
Cell Death & Disease (2022)
-
c-FLIP promotes drug resistance in non-small-cell lung cancer cells via upregulating FoxM1 expression
Acta Pharmacologica Sinica (2022)
-
BRCA1 mutations in high-grade serous ovarian cancer are associated with proteomic changes in DNA repair, splicing, transcription regulation and signaling
Scientific Reports (2022)