Abstract
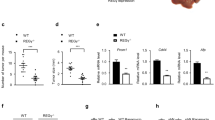
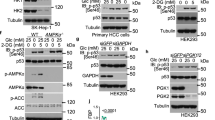
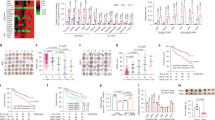
Phosphoenolpyruvate carboxykinase (PEPCK or PCK) catalyzes the first rate-limiting step in hepatic gluconeogenesis pathway to maintain blood glucose levels. Mammalian cells express two PCK genes, encoding for a cytoplasmic (PCPEK-C or PCK1) and a mitochondrial (PEPCK-M or PCK2) isoforms, respectively. Increased expressions of both PCK genes are found in cancer of several organs, including colon, lung, and skin, and linked to increased anabolic metabolism and cell proliferation. Here, we report that the expressions of both PCK1 and PCK2 genes are downregulated in primary hepatocellular carcinoma (HCC) and low PCK expression was associated with poor prognosis in patients with HCC. Forced expression of either PCK1 or PCK2 in liver cancer cell lines results in severe apoptosis under the condition of glucose deprivation and suppressed liver tumorigenesis in mice. Mechanistically, we show that the pro-apoptotic effect of PCK1 requires its catalytic activity. We demonstrate that forced PCK1 expression in glucose-starved liver cancer cells induced TCA cataplerosis, leading to energy crisis and oxidative stress. Replenishing TCA intermediate α-ketoglutarate or inhibition of reactive oxygen species production blocked the cell death caused by PCK expression. Taken together, our data reveal that PCK1 is detrimental to malignant hepatocytes and suggest activating PCK1 expression as a potential treatment strategy for patients with HCC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Hsu PP, Sabatini DM. Cancer cell metabolism: Warburg and beyond. Cell. 2008;134:703–7.
Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–33.
Warburg O. On the origin of cancer cells. Science. 1956;123:309–14.
Burgess SC, He T, Yan Z, Lindner J, Sherry AD, Malloy CR, et al. Cytosolic phosphoenolpyruvate carboxykinase does not solely control the rate of hepatic gluconeogenesis in the intact mouse liver. Cell Metab. 2007;5:313–20.
Yang J, Kalhan SC, Hanson RW. What is the metabolic role of phosphoenolpyruvate carboxykinase? J Biol Chem. 2009;284:27025–9.
Méndez-Lucas A, Duarte JA, Sunny NE, Satapati S, He T, Fu X, et al. PEPCK-M expression in mouse liver potentiates, not replaces, PEPCK-C mediated gluconeogenesis. J Hepatol. 2013;59:105–13.
Montal ED, Dewi R, Bhalla K, Ou L, Hwang BJ, Ropell AE, et al. PEPCK coordinates the regulation of central carbon metabolism to promote cancer cell growth. Mol Cell. 2015;60:571–83.
Vincent EE, Sergushichev A, Griss T, Gingras MC, Samborska B, Ntimbane T, et al. Mitochondrial phosphoenolpyruvate carboxykinase regulates metabolic adaptation and enables glucose-independent tumor growth. Mol Cell. 2015;60:195–207.
Leithner K, Hrzenjak A, Trötzmüller M, Moustafa T, Köfeler HC, Wohlkoenig C, et al. PCK2 activation mediates an adaptive response to glucose depletion in lung cancer. Oncogene. 2014;34:1044–50.
Sunny NE, Parks EJ, Browning JD, Burgess SC. Excessive hepatic mitochondrial TCA cycle and gluconeogenesis in humans with nonalcoholic fatty liver disease. Cell Metab. 2011;14:804–10.
Satapati S, Kucejova B, Duarte JA, Fletcher JA, Reynolds L, Sunny NE, et al. Mitochondrial metabolism mediates oxidative stress and inflammation in fatty liver. J Clin Invest. 2015;125:4447–62.
Owen OE, Kalhan SC, Hanson RW. The key role of anaplerosis and cataplerosis for citric acid cycle function. J Biol Chem. 2002;277:30409–12.
Beale EG, Harvey BJ, Forest C. PCK1 and PCK2 as candidate diabetes and obesity genes. Cell Biochem Biophys. 2007;48:89–95.
Hirayama A, Kami K, Sugimoto M, Sugawara M, Toki N, Onozuka H, et al. Quantitative metabolome profiling of colon and stomach cancer microenvironment by capillary electrophoresis time-of-flight mass spectrometry. Cancer Res. 2009;69:4918–25.
Dunten P, Belunis C, Crowther R, Hollfelder K, Kammlott U, Levin W, et al. Crystal structure of human cytosolic phosphoenolpyruvate carboxykinase reveals a new GTP-binding site. J Mol Biol. 2001;316:257–64.
Balan MD, McLeod MJ, Lotosky WR, Ghaly M, Holyoak T. Inhibition and allosteric regulation of monomeric phosphoenolpyruvate carboxykinase by 3-mercaptopicolinic acid. Biochemistry. 2015;54:5878–87.
Burgess SC, Hausler N, Merritt M, Jeffrey FM, Storey C, Milde A, et al. Impaired tricarboxylic acid cycle activity in mouse livers lacking cytosolic phosphoenolpyruvate carboxykinase. J Biol Chem. 2004;279:48941–9.
Hakimi P, Johnson MT, Yang J, Lepage DF, Conlon RA, Kalhan SC, et al. Phosphoenolpyruvate carboxykinase and the critical role of cataplerosis in the control of hepatic metabolism. Nutr Metab. 2005;2:33.
Hitosugi T, Zhou L, Elf S, Fan J, Kang HB, Seo JH, et al. Phosphoglycerate mutase 1 coordinates glycolysis and biosynthesis to promote tumor growth. Cancer Cell. 2012;22:585–600.
Sullivan LB, Martinez-Garcia E, Nguyen H, Mullen AR, Dufour E, Sudarshan S, et al. The proto-oncometabolite fumarate binds glutathione to amplify ROS-dependent signaling. Mol Cell. 2013;51:236–48.
Zheng L, Cardaci S, Jerby L, MacKenzie ED, Sciacovelli M, Johnson TI, et al. Fumarate induces redox-dependent senescence by modifying glutathione metabolism. Nat Commun. 2015;6:6001.
Jin L, Li D, Alesi GN, Fan J, Kang HB, Lu Z, et al. Glutamate dehydrogenase 1 signals through antioxidant glutathione peroxidase 1 to regulate redox homeostasis and tumor growth. Cancer Cell. 2015;27:257–70.
Sabharwal SS, Schumacker PT. Mitochondrial ROS in cancer: initiators, amplifiers or an Achilles’ heel? Nat Rev Cancer. 2014;14:709–21.
Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1–13.
Shen S, Guo X, Yan H, Lu Y, Ji X, Li L, et al. A miR-130a-YAP positive feedback loop promotes organ size and tumorigenesis. Cell Res. 2015;25:997–1012.
Hernandez-Gea V, Toffanin S, Friedman SL, Llovet JM. Role of the microenvironment in the pathogenesis and treatment of hepatocellular carcinoma. Gastroenterology. 2013;144:512–27.
Tlsty TD, Coussens LM. Tumor stroma and regulation of cancer development. Annu Rev Pathol. 2006;1:119–50.
Mao Y, Keller ET, Garfield DH, Shen K, Wang J. Stromal cells in tumor microenvironment and breast cancer. Cancer Metastas- Rev. 2013;32:303–15.
Li B, Qiu B, Lee DS, Walton ZE, Ochocki JD, Mathew LK, et al. Fructose-1,6-bisphosphatase opposes renal carcinoma progression. Nature. 2014;513:251–5.
Yang H, Zhou L, Shi Q, Zhao Y, Lin H, Zhang M, et al. SIRT3-dependent GOT2 acetylation status affects the malate-aspartate NADH shuttle activity and pancreatic tumor growth. EMBO J. 2015;34:1110–25.
Ma S, Jiang B, Deng W, Gu ZK, Wu FZ, Li T, et al. D-2-hydroxyglutarate is essential for maintaining oncogenic property of mutant IDH-containing cancer cells but dispensable for cell growth. Oncotarget. 2015;6:8606–20.
Haslene-Hox H, Oveland E, Berg KC, Kolmannskog O, Woie K, Salvesen HB, et al. A new method for isolation of interstitial fluid from human solid tumors applied to proteomic analysis of ovarian carcinoma tissue. PLoS ONE. 2011;6:e19217.
Acknowledgements
We thank Dr Bin Zhao (Zhejiang University, Hangzhou, China) for providing plasmids used for inducing liver tumor in mice, including PB[CMV-myc-YAP-5SA]DS, PB[Act-RFP]DS, and Act-PB Transposase. We thank Xiaocan Guo for technical help on hydrodynamic injection. We also thank Dr Jiahuai Han (Xiamen University, Xiamen, China) for generously offering cDNAs for human PCK1 and PCK2 genes. This work was supported by the National Science and Technology Major Project of China (No. 2017ZX10203207-002-004 to HXY), the 973 Program (No. 2015CB910401 to YX), the NSFC grants (No. 31570784 and 81773190 to HXY), and NIH grants (CA163834 to YX; CA196878 and GM51586 to K-LG).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
K-LG is co-founder of Vivace Therapeutics. The remaining authors declare that they have no competing interests.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Liu, MX., Jin, L., Sun, SJ. et al. Metabolic reprogramming by PCK1 promotes TCA cataplerosis, oxidative stress and apoptosis in liver cancer cells and suppresses hepatocellular carcinoma. Oncogene 37, 1637–1653 (2018). https://doi.org/10.1038/s41388-017-0070-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-017-0070-6
This article is cited by
-
Nuclear respiratory factor 1 regulates super enhancer-controlled SPIDR to protect hepatocellular carcinoma cells from oxidative stress
BMC Gastroenterology (2024)
-
Upregulation of p300 in paclitaxel-resistant TNBC: implications for cell proliferation via the PCK1/AMPK axis
The Pharmacogenomics Journal (2024)
-
Expression and functional significance of phosphoenolpyruvate carboxykinase 1 in uveal melanoma
Cell Death Discovery (2024)
-
CDCA8 Facilitates Tumor Proliferation and Predicts a Poor Prognosis in Hepatocellular Carcinoma
Applied Biochemistry and Biotechnology (2024)
-
Energy metabolism as the hub of advanced non-small cell lung cancer management: a comprehensive view in the framework of predictive, preventive, and personalized medicine
EPMA Journal (2024)