Abstract
The optimal dietary regimen for polycystic ovary syndrome (PCOS) has not been identified. High-protein diets (HPDs) are effective for weight control in individuals with metabolic abnormalities, but no systematic meta-analyses have yet summarised the effects of HPDs on PCOS. Seven electronic databases were searched from inception to 30 April 2023, and studies comparing the effects of HPDs and other diets on the anthropometrics, metabolic factors, and hormonal profiles for PCOS were identified. Data were pooled using random-effects models and expressed as weighted mean differences and 95% confidence intervals. The risk of bias was assessed by Cochrane Collaboration tool. Eight trials involving 300 women with PCOS were included. Compared with isocaloric balanced diets (BDs), HPDs significantly reduced fasting insulin (−2.69 μIU/mL, 95% CI [−3.81, −1.57], P < 0.0001, I2 = 46%) and homoeostatic model assessment for insulin resistance (HOMA-IR−0.41, 95% CI [−0.80, −0.02], P = 0.04, I2 = 94%) in women with PCOS. However, HPDs and BDs had comparable effects on weight loss, abdominal adiposity, lipid profiles, and reproductive hormones (all P ≥ 0.05). HPDs may benefit women with PCOS in terms of improving insulin resistance, supporting for their use as one of the dietary management options for PCOS, however further RCTs in larger and broader settings are required to confirm these observations and investigate the mechanism behind it.
Similar content being viewed by others
Introduction
Polycystic ovary syndrome (PCOS), a syndrome characterized by hyperandrogenism, menstrual irregularities, and polycystic ovarian morphology [1], is a common endocrine disorder and a primary cause of anovulation in up to 18% of women of reproductive age. Women with PCOS often exhibit metabolic abnormalities, including insulin resistance (IR), hyperinsulinaemia, and obesity. They are also at an increased risk of developing metabolic syndrome and type 2 diabetes mellitus (T2DM) [2, 3]. IR and hyperinsulinaemia are key pathophysiological factors linked to a series of metabolic and reproductive disorders in women with PCOS [4] and can be exacerbated by overweight or obesity [5]. These conditions affect ovarian function by interacting with gonadotropins, which results in the overproduction of ovarian androgen and prevents ovulation [6].
Weight loss is one of the primary therapies for PCOS. For patients with IR or hyperandrogenism, even a modest weight loss of 5% may have positive effects, such as restoring their regular menses and improving their response to ovulation-induction and fertility medications [7,8,9]. According to the International Evidence-Based Guidelines for the Assessment and Management of Polycystic Ovary Syndrome, lifestyle modifications such as dietary interventions are recommended as first-line therapy for managing the metabolic complications of PCOS [10]. However, the success and sustainability of weight loss diets have been the subject of debate. According to the literature, women with PCOS tend to be obese [2], do not fully comply with energy-restricted diets [11], and have difficulty in maintaining their weight after weight loss [12, 13], which may be attributed to psychosocial, physiological, or appetite regulatory factors [14, 15]. Therefore, understanding the most useful types and components of diets is essential for the success and sustainability of management strategies that target healthy pregnancies and lifelong health among women with PCOS. Energy restriction, intermittent fasting, high-protein diets (HPDs), low-glycemic index diets, and Mediterranean diets are some of the most effective approaches for weight loss. Of these approaches, HPDs are considered the most effective [16,17,18], especially their function in improving IR [18].
In terms of muscle and body composition, women with PCOS have a smaller amount of lean body tissue than that of healthy women [19]. Muscles are a crucial endocrine organ. Inadequate muscle mass may reduce the number of insulin receptors and affect the metabolism of glucose and lipids [20]. HPDs can increase muscle mass, thereby improving the control of blood glucose, lipids, and IR. A study indicated that inflammation is one of the most crucial yet overlooked risk factors for PCOS [21], and high-protein intake and improved muscle mass can help improve the inflammatory status of the body [22].
HPDs are widely used for weight control. Although studies have investigated the use of HPDs for patients with PCOS, their results have been inconsistent. Therefore, we conducted a systematic review and meta-analysis to investigate whether HPDs are useful for improving IR, body weight, and glucolipid metabolism in women with PCOS. We also summarised the adverse effects associated with high-protein intake in studies.
Materials and methods
This meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [23].
PICOTS
PICOTS (Population [P], Intervention [I], Comparison [C], Outcome [O], Time [T], and Study [S]) was defined before the study search process. Our research question was whether according to RCTs (S), HPDs (I) compared with isocaloric balanced diets (BDs; C) can lead to improved metabolic and reproductive-health outcomes (O) in patients with PCOS (P). Further details regarding the PICOTS criteria are provided in Supplementary Table 1. Because IR and hyperinsulinaemia are major causes of PCOS, we used homoeostatic model assessment for insulin resistance (HOMA-IR) and fasting insulin (FINS) levels as glucose metabolism indicators; total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and triglycerides (TGs) as lipid metabolism indicators; and body weight, waist circumference (WC), total testosterone (TT), dehydroepiandrosterone sulphate (DHEAS), sex hormone binding globulin (SHBG), and the free androgen index (FAI) as observation indicators.
Databases and search strategy
Seven electronic databases, namely MEDLINE (PubMed), Embase, ClinicalTrials.gov, Web of Science, China National Knowledge Infrastructure, Wanfang Data, and Cochrane Library, were searched from inception to 30 April 2023 to identify studies comparing the effects of HPDs and other diets on the IR, anthropometrics, lipid profiles, glucoregulatory outcomes, and hormonal profiles of women with PCOS. The following PCOS-related search terms were used: polycystic ovary syndrome OR polycystic ovar* OR poly-cystic ovar* OR PCOS OR PCO* OR leventhal OR anovulation OR anovulat* OR oligo-ovulat* OR oligoovulat* OR sclerocystic ovary syndrome. In addition, the following diet-related keywords were used: high protein OR high-protein low-carbohydrate OR high protein intake OR carbohydrate-restricted OR diet composition OR high protein. Further details regarding the search strategy are provided in Supplementary Table 2. Reference lists and conference proceedings were manually examined to obtain additional relevant data. The language was restricted to English and Chinese.
Study selection
The following types of studies were included: (1) studies focusing on women with PCOS; (2) studies evaluating the effects of HPDs and other isocaloric diets, with the proportion of energy supplied by protein representing at least 25% of the total dietary energy intake; (3) parallel or cross-over RCTs; (4) studies with an intervention duration of 4 weeks or more; and (5) studies in which the outcomes included at least one of the following: weight, body mass index (BMI), WC, waist-to-hip ratio (WHR), FINS, HOMA-IR, reproductive hormones, and lipid profile. The following types of studies were excluded: (1) cohort or case–control studies, reviews, meta-analyses, case reports, and animal or cell experiments; (2) studies focusing on pregnancy or lactation; (3) studies involving women with other causes for hyperandrogenism and abnormal ovulation or any serious medical, psychiatric, or neurological problems; and (4) studies with missing data.
Data extraction
Literature screening was independently conducted by two investigators (FW and PJL). All discrepancies and disagreements regarding study inclusion and exclusion were resolved by consensus or consultation with a third investigator (WW). Basic data were collected using a predesigned data extraction form and included the general characteristics of each study, that is, the (1) name of the first author, year of publication, and country of study; (2) participant characteristics, including the total sample size and actual sample size, age at baseline, duration of intervention, and criteria used to define PCOS; (3) study design and duration of intervention; (4) dietary characteristics, including type, energy, macronutrient composition, ratio, and specific forms of dietary control; and (5) baseline and postintervention metabolic and reproductive outcomes, including weight, BMI, fasting plasma glucose (FPG), glycosylated haemoglobin, TG, LDL-C, TC, HDL-C, HOMA-IR, FINS, TT, DHEAS, SHBG, and the FAI. The completion rates, adverse events, and whether any other interventions were implemented were recorded and assessed. Data were extracted by PJL and examined by FW for any potential errors.
Quality assessment
Methodological quality was independently evaluated by two researchers (FW and PJL) by using the Cochrane Collaboration tool [24]. All disagreements were resolved through consultation with a third researcher (WW). Studies were evaluated as having low or high bias or unclear risk on the basis of the following: sequence generation, allocation concealment, participant blinding, personnel and outcome assessors, outcome assessment blinding, incomplete outcome data, selective outcome reporting, and other types of bias.
Data analysis
All statistical analyses were conducted using STATA 14.0 (StataCorp, College Station, TX, USA). Changes in each outcome were reported as differences between mean values before and after the intervention. If the means and standard deviations (SDs) of changes from baseline were specified in the papers, they were directly used. If not, mean changes in the observed parameters were calculated by subtracting the baseline values from the postintervention values. The SD of each difference was calculated as follows:
If the target data in an included study were expressed as medians (quantile interval), the mean value of the target data was determined using the method of Luo et al. [25] or Hozo et al. [26] when appropriate. In brief, if the sample size in an included study is >25, then the median is considered as the mean value of target data; while the sample size in an included study is less than or equal to 25, the estimated average value is calculated using the following formula based on the data format given in the included study:
Mean = (a + 2*m + b)/4 OR Mean = (q1 + m + q3)/3 [a, b, and m represent the minimum, maximum, and median values of the target data, respectively; q1 or q3 represents the cut-off value of the first or third quantile of the target data, respectively].
In addition, depending on the characteristics of the included data, the SDs of the target data were calculated using the method of Wan et al. [27] or Hozo et al. [26]. In short, the estimated SDs of the target data were calculated as (b − a) / 4 [the sample size of the included study is <70] or (b − a) / 6 [the sample size of the included study is of ≥70] {a and b represent the minimum, maximum values of the target data, respectively}
Subsequently, the results were pooled for meta-analysis as mean differences with 95% confidence intervals (CIs). Statistical significance was set at P < 0.05. The heterogeneity within comparisons was evaluated using Cochran’s Q test and quantified using the I2 statistic. I2 values of <25%, 25%–50%, and >50% represented low, moderate, and high heterogeneity, respectively [28].
A random-effects method was used to calculate summary effect measures at I2 > 0%. Sensitivity analysis was then conducted by including only studies with a low risk of bias. Subgroup analysis was conducted in cases involving more than three studies. The subgroups were categorised on the basis of the intervention duration (<12 and ≥12 weeks) and ethnicity. When required, Egger’s test and funnel plots were used to investigate potential publication bias [29, 30].
Results
Study characteristics
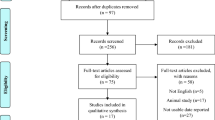
In the preliminary search, 853 studies were identified. We identified 10 articles reporting on 8 RCTs that involved a total of 300 participants (154 in the intervention group and 146 in the control group) as eligible for meta-analysis [31,32,33,34,35,36,37,38,39,40]. Further details regarding the selection process are provided in the PRISMA flow diagram in Fig. 1.
Table 1 presents the general characteristics of the included studies, which were published between 2003 and 2021. These studies were parallel-design, single-centre trials conducted in the United States [32, 37], Australia [33, 34], Brazil [31], Iran [35, 36, 38], and China [38, 39]. All studies included women with overweight and obesity (BMI ≥ 24 kg/m2), except one [31] recruited patients without BMI limitations. The participants of the Toscani study [31] were aged between 14–35, whereas those in other studies were between 18–45 years old. All participants received a diagnosis of PCOS based on the criteria of the National Institutes of Health (NIH) [32,33,34,35, 37], Rotterdam consensus [36,37,38], or Androgen Excess and Polycystic Ovary Syndrome (AE-PCOS) [31], with one participant not receiving a diagnosis [40]. The intervention duration ranged from 4–16 weeks. All studies focused on HPDs as an exposure factor and compared the effects of HPDs and isocaloric BDs. Three studies used protein powder as a protein administration method for the HPD intervention group [36, 38, 39], whereas the other studies used a high-protein dietary pattern as an intervention measure.
Meta-analysis results
Anthropometrics
Weight
According to data pooled from seven of the eligible studies [31,32,33, 35, 37,38,39], HPDs did not significantly reduce body weight when compared with BDs (−0.78 kg, 95% CI [−1.69, 0.13], P = 0.06; Fig. 2A). The degree of heterogeneity was high (I2 = 82%). Removing any of the seven studies did not cause the I2 to decrease to below 50%.
When conducting subgroup analysis by country, after a Chinese study was excluded [39], no significant changes were observed in the results (−0.83 kg, 95% CI [−1.75, 0.09], P = 0.08, I2 = 86%). According to the subgroup analysis results for intervention duration, HPDs were not superior to BDs in terms of leading to weight loss when they were implemented as short-term (<12 weeks) [31, 32, 37, 39] or long-term (≥12 weeks) [33, 35, 38] interventions (P = 0.42 and 0.09, respectively), with high heterogeneity noted in the subgroups (82% and 77%). In addition, according to the subgroup analysis results for ethnicity, HPDs and BDs had similar effects (P = 0.07 and 0.65, respectively; Supplementary Table 3).
BMI
According to data pooled from five eligible studies [33, 37,38,39,40], when compared with BDs, HPDs did not significantly reduce BMI (−0.81 kg/m2, 95% CI [−1.69, 0.07], P = 0.07; Fig. 2B). The degree of heterogeneity was high (I2 = 83%). After the study by Nadjarzadeh et al. [38] was excluded, sensitivity analysis revealed a change in the overall effect size (−1.36, 95% CI [−1.63, −1.08], P < 0.0001, I2 = 0).
After the two Chinese studies [39, 40] were excluded, no significant changes were observed in the results (−0.42 kg/m2, 95% CI [−2.06, 1.21], P = 0.61, I2 = 89%). In terms of intervention duration, HPDs did not significantly reduce BMI when implemented as long-term interventions (−0.58 kg/m2, 95% CI [−2.55, 1.38], P = 0.56, I2 = 90%) [33, 38, 40]. According to the subgroup analysis results for ethnicity, HPDs and BDs had similar effects on Asian populations (P = 0.47, Supplementary Table 3).
Abdominal obesity
Three studies [32, 38, 40] reported a change in WHR (−0.02, 95% CI [−0.04, 0.01], P = 0.16, I2 = 0%; Fig. 2C). Similarly, three studies [31, 32, 38] reported a change in WC (−2.56 cm, 95% CI [−5.91, 0.79], P = 0.13, I2 = 0%; Fig. 2D). These results indicate that both interventions similarly reduced WC and WHR. However, because the number of studies reporting such data was limited, no subgroup analysis was conducted.
Glucoregulatory indicators
FINS
According to data pooled from seven eligible studies [31, 33, 35,36,37, 39, 40], when compared with BDs, HPDs significantly reduced concentrations of FINS (−2.69 mIU/mL, 95% CI [−3.81, −1.57], P < 0.00001, I2 = 46%; Fig. 3A). Removing any of these studies did not decrease the I2 to be <50%. After the two Chinese studies [39, 40] were excluded, no significant changes were observed in the results (−2.41 mIU/mL, 95% CI [−3.6, −1.21], P < 0.0001, I2 = 54%).
In the long-term studies (≥12 weeks) [33, 35, 36, 40], the concentrations of FINS were significantly lower in the HPD group than in the BD group (−3.04 mIU/mL, 95% CI [−4.56, −1.51], P < 0.0001, I2 = 56%). However, in the short-term studies [31, 37, 39], HPDs and BDs had similar effects (−2.58, 95% CI [−5.66, 0.51], P = 0.1, I2 = 55%). In the studies focusing on FINS levels, with the exception of the study by Toscani et al. [31], all participants were women with overweight or obesity. According to the pooled data on the women with PCOS in these studies, the concentration of FINS significantly decreased after HPD interventions (−2.86 mIU/mL, 95% CI [−4.17, −1.54], P < 0.0001, I2 = 59%). The results of the subgroup analysis were consistent between the studies involving Asian populations and studies involving European and American populations, with these groups having effect sizes of −2.88 mIU/mL (95% CI [−5.02, −0.73), P = 0.009) and −2.49 mIU/mL (95% CI [−4.73, −0.24], P = 0.003), respectively (Supplementary Table 3).
FPG
Six studies [31, 34, 36, 37, 39, 40] reported changes in FPG levels before and after the intervention. Pooled analysis revealed a significantly higher concentration of FPG (2.33 mg/dL, 95% CI [0.63, 4.03], P = 0.007, I2 = 10%) in the HPD group than in the BD group (Fig. 3B). After the two Chinese studies [39, 40] were excluded, no significant changes were observed in the results [2.68 mg/dL, 95% CI [0.93, 4.43], P = 0.003, I2 = 0%]. In terms of intervention duration, subgroup analysis revealed that compared with BDs, HPDs were associated with higher concentrations of FPG (3.19 mg/dL, 95% CI [0.97, 5.42], P = 0.005, I2 = 0%) in short-term studies [31, 37, 39]. However, in long-term studies (≥12 weeks) [34, 36, 40], HPDs and BDs had similar effects on the concentrations of FPG [0.39 mg/dL, 95% CI [−5.39, 6.17], P = 0.89, I2 = 47%]. These results indicate that HPD interventions lasting 8 weeks or less may be associated with higher concentrations of FPG than those associated with BD interventions lasting 8 weeks or less. However, for interventions lasting 12 weeks or more, HPDs and BDs have similar effects (Supplementary Table 3).
According to the subgroup analysis results for ethnicity, HPDs significantly increased the concentrations of FPG among European and American populations (2.92 mg/dL, 95% CI [1.12, 4.72], P = 0.001, I2 = 0%) but not among Asian populations (−2.54 mg/dL, 95% CI [−7.72, 2.64], P = 0.34, I2 = 0%).
HOMA-IR
According to data pooled from six eligible studies [31, 33,34,35,36,37,38,39], HPDs and BDs had different effects on HOMA-IR (−0.41, 95% CI [−0.78, −0.03], P = 0.03; Fig. 3C). The degree of heterogeneity was high (I2 = 96%). After the two studies by Moran et al. [33, 34] were excluded (I2 = 39%), no significant changes were observed in the results (−0.58, 95% CI [−0.95, −0.20], P = 0.003).
In the long-term studies [33,34,35,36, 38], HPDs and BDs had similar effects on HOMA-IR (−0.20, 95% CI [−0.65, 0.25], P = 0.38, I2 = 97%). However, in the short-term studies [31, 37, 39], HPDs resulted in a significantly larger reduction in HOMA-IR (−0.82, 95% CI [−1.29, −0.35], P = 0.0006, I2 = 35%; Supplementary Table 3).
Blood lipid profiles
TC
Six studies [31, 32, 34, 35, 37, 40] reported changes in TC. Meta-analysis revealed no significant difference between the changes in TC resulting from HPDs and BDs in these studies (−10.68 mg/dL, 95% CI [−24.57, 3.21], P = 0.13, I2 = 94%; Fig. 4A). Subgroup analysis revealed no significant difference in the effects of HPDs and BDs on TC in either short-term (<12 weeks) or long-term (≥12 weeks) interventions (P = 0.06 and 0.79, respectively; Supplementary Table 3). Removing any of these studies did not cause the I2 to decrease to below 50%.
TG levels
Six studies [31, 32, 34, 35, 37, 40] reported changes in TG levels. Pooled analysis revealed no significant difference between the influence of HPDs and BDs on these levels (−17.13 mg/dL, 95% CI [−35.37, 1.11], P = 0.07, I2 = 88%; Fig. 4B). After a Chinese study [40] was excluded, no significant changes were observed in the results (P = 0.12). In the short-term studies (4–8 weeks) [31, 32, 37], HPDs were significantly more effective than BDs were in reducing TG levels (−31.38 mg/dL, 95% CI [−41.77, −21.00], P < 0.0001, I2 = 0%). However, in the long-term studies (≥12 weeks) [34, 35, 40], no significant difference was observed in the effects of HPDs and BDs on TG reduction (Supplementary Table 3). Even when the studies were removed one by one, the degree of heterogeneity remained high.
HDL-C
Six studies [31, 32, 34, 35, 37, 40] reported changes in HDL-C. Their results revealed no significant difference between the influence of HPDs and BDs on HDL-C (1.94 mg/dL, 95% CI [−1.82, 5.69], P = 0.31, I2 = 95%; Fig. 4C). According to the subgroup analysis results for intervention duration, similar outcomes were observed in the short- and long-term studies (P = 0.46 and 0.08, respectively; Supplementary Table 3). Even when studies were removed one by one, the degree of heterogeneity remained high.
LDL-C
Four studies [31, 32, 34, 35] reported changes in LDL-C. Their results revealed no significant difference between the influence of HPDs and BDs on LDL-C (−1.80 mg/dL, 95% CI [−4.03, 0.44], P = 0.11, I2 = 0%; Fig. 4D). Because the number of studies reporting on LDL-C was limited, no subgroup analysis was conducted.
Reproductive hormones
TT
According to data pooled from five eligible studies [32, 35, 37,38,39], HPDs did not significantly reduce concentrations of TT (−0.20 nmol/L, 95% CI [−0.50, 0.10], P = 0.20) compared with isocaloric BDs (Fig. 5A). The degree of heterogeneity was high (I2 = 84%, P < 0.0001). After the study by Nadjarzadeh et al. [38] was excluded, the I2 decreased to 47%, with an effect size of −0.39 nmol/L (95% CI [−0.71, −0.07], P = 0.02). Subgroup analysis revealed no significant difference in the effects of HPDs and isocaloric BDs on the concentrations of TT, regardless of intervention duration and ethnicity (Supplementary Table 3).
DHEAS
According to data pooled from three eligible studies [32, 35, 37], HPDs did not significantly reduce concentrations of DHEAS (7.67 ng/mL, 95% CI [−38.78, 54.11], P = 0.75, I2 = 87%) compared with isocaloric BDs (Fig. 5B).
FAI
According to data pooled from five eligible studies [33, 35, 37,38,39], no significant differences were observed in the effects of HPDs and isocaloric BDs on the FAI (−0.07, 95% CI [−0.81, 0.66], P = 0.84, I2 = 75%; Fig. 5C). According to the subgroup analysis results for three long-term studies [33, 35, 38], HPDs and isocaloric BDs had similar effects on the FAI (0.37, 95% CI [−0.84, 1.57], P = 0.55, I2 = 29%). Additionally, according to the combined results of three studies [35, 38, 39], HPDs and BDs had similar effects on the Asian populations (P = 0.24, Supplementary Table 3).
SHBG
Four studies reported changes in the concentrations of SHBG [35, 37,38,39]. Pooled analysis revealed that neither HPDs nor isocaloric BDs significantly reduced the concentrations of SHBG after the intervention (6.20 nmol/L, 95% CI [−2.90, 15.29], P = 0.18, I2 = 92%; Fig. 5D). However, because the number of studies reporting concentrations of SHBG was limited, no subgroup analysis was conducted.
Study quality and risk of bias
A quality assessment of each trial is presented in Fig. 6A and B, and the details are provided in Supplementary Table 4. Four studies [32, 38,39,40] provided detailed information regarding their randomisation methods, and five studies [31, 33, 35, 37, 38] explained their methods of allocation concealment. The risk of intervention blinding was high in three studies [34, 39, 40], low in three studies [35, 37, 38], and unclear in two studies [31, 32]. Data analysis blinding was performed in all studies. Five studies [32, 34, 35, 37, 39] had a high risk of reporting bias, and three studies [31, 38, 40] had a low risk of reporting bias.
Discussion
Diet control is an essential technique for achieving or maintaining the optimal weight for women with PCOS, although no consensus has yet been reached regarding the optimal distribution of dietary components for such women [19]. Multiple studies have indicated that HPDs have positive effects on obesity, metabolic syndrome, and diabetes [16,17,18]. However, no systematic meta-analysis has specifically summarised the effects of HPDs on PCOS. We reviewed eight RCTs involving a total of 300 women. Our results indicate that, compared with BDs, HPDs are associated with a significantly greater reduction in FINS and HOMA-IR levels in patients with PCOS. However, HPDs and BDs have similar effects on weight, abdominal obesity, lipid profiles, and sex hormone levels.
Our finding, that HPDs and BDs with the same energy restrictions have similar effects on body weight, BMI, and abdominal obesity, is consistent with the previous studies with similar intervention length [34, 41,42,43]. Collectively, it appears that when energy intake is controlled, the distribution of macronutrients does not affect the amount of weight loss. In the current study, we noted a high degree of heterogeneity in the pooled results of weight and BMI among the included studies, which can be explained by differences in the durations of interventions and the ethnicities. Some studies have indicated extending HPD interventions (e.g., 4 weeks or more) may promote the loss of body weight and fat and improve subsequent weight maintenance [41, 44]. Our subgroup analysis revealed a long-term intervention of 12 weeks or more increased the trend of weight loss, although the results did not reach statistical significance. The mechanism underlying the effects of HPDs on weight loss is likely linked to high energy expenditure. Compared to BDs, HPDs could maintain or increase lean body mass [45], which is a predominant contributor to resting energy expenditure (REE). The HPDs were found to bring less REE reduction during weight loss whereas others did not [46]. Moreover, protein consumption has higher dietary thermogenesis than carbohydrates or fat due to different nutrient processing [47]. HPDs also contribute to a greater satiating effect and the following reduction in food consumption and weight [48]. One of the explanations for such satiety is the increased levels of anorexigenic hormones, such as glucagon-like peptide-1, cholecystokinin, and peptide tyrosine-tyrosine [49, 50]. These hormones and vagal afferent fibres can work on brain regions related to reward and motivation, hypothalamus, and other regions responsible for energy homoeostasis, and regulate people’s dietary consumption and adherence to weight control programmes [51]. Our results for weight and BMI showed the tendency for HPD to lead to more reduction of weight and BMI (P = 0.06 and 0.07), however, since total energy intake prescribed in HP and control groups were comparable and low in most studies, the difference was not significant. It may also be possible that a 4–12 weeks intervention is not long enough to see the difference in adherence or the effect of satiety from HPD on total energy intake. The lower spontaneous energy intake led by satiating effect and adherence of HPD might be seen when there is free dietary choice rather than isoenergetic with the comparative diet. Although no changes in fat mass were reported in the included studies, they used WC and WHR to assess the abdominal fat improvement, and the results revealed that HPDs did not significantly reduce abdominal obesity. Therefore, current evidence indicates that different diets with isoenergetic restriction can promote similar weight loss and the calorie deficit is fundamental for weight control.
In terms of IR, we discovered HPDs were more effective than BDs in improving the levels of FINS and HOMA-IR in patients with PCOS. Subgroup analysis revealed that HPDs and isoenergetic BDs had a similar effect on the concentration of FINS in short-term interventions (8 weeks or less) whereas that HPDs offer additional advantages in long-term interventions (12 weeks or more). Subgroup analysis of ethnicity also revealed an advantage of HPDs in terms of FINS reduction both in European, American, and Asian populations. Our findings are consistent with those who reported that HPD interventions improved the FINS and insulin homoeostasis [18, 42, 52]. Protein and amino acid intake are known to enhance insulin secretion, which is associated with a more than compensatory increase in insulin clearance, thus resulting in lower plasma insulin levels [53, 54]. Moreover, HPDs is with a corresponding reduction of carbohydrates, which could improve insulin sensitivity, enhance pancreatic β-cell function and endogenous insulin clearance [55]. Some studies conducted among participants with overweight or obesity observed a larger reduction of FINS with HPD than average-protein diets, although there were similar effects on weight and FPG change [56]. Our analysis revealed a mild reduction in HOMA-IR, with this reduction primarily attributable to the findings of Dou et al. [39]. When this study was excluded, pooled analysis revealed the two interventions had similar effects on HOMA-IR. Because HOMA-IR here was calculated using a formula instead of a hyperinsulinaemic (euglycaemic) clamp [57], the value was influenced by both FPG and FINS. According to a previous study [58], insulin levels change more rapidly than FPG levels do, which may explain the substantial reductions observed in FINS and HOMA-IR in the current analysis. Overall, these findings indicate that compared with BDs, HPDs are associated with considerably more favourable insulin and HOMA-IR.
We investigated the effects of HPDs on the metabolism of glucolipids and observed a slight increase in FPG levels when HPDs were implemented (2.33 mg/dL, 95% CI [0.63, 4.03], P = 0.007, I2 = 10%). As an indicator of instantaneous blood glucose, FPG is influenced by various factors and cannot be solely relied upon as an indicator of blood glucose control. FPG findings are also typically influenced by the level of glucose at baseline, the source of protein, and the study duration. The effect of HPD on lipids is mostly through weight loss. Although the short-term studies analyzed in the current meta-analysis revealed a favourable effect of HPDs on TG reduction, this advantage was not observed in the long-term studies, which is presumably because of the comparable energy balance and fat content between the groups, so as the similar weight and fat reduction in these studies.
We noted both a decrease in the FAI and levels of TT and an increase in the levels of SHBG and DHEAS in the patients with PCOS, regardless of their dietary patterns. These findings are consistent with those of one previous study indicating that even modest weight loss of 5%–10% over a short duration of 4 weeks may lead to improvements in PCOS symptoms [32], including those participants who still had obesity or overweight. Thus, regardless of whether a high-protein model is adopted, energy restriction is essential for achieving hormonal improvements. However, HPDs did not show an advantage in modulating endocrine hormones compared to BDs, which may have resulted from similar weight loss or the short duration of the intervention.
During the data merging process, we encountered a high degree of heterogeneity. To explore the potential effects of such heterogeneity on our results, we conducted several subgroup analyses involving factors such as baseline BMI category, ethnicity, and intervention duration. Nevertheless, heterogeneity persisted in the data, presumably because of the inherent complexity of patients with PCOS, who exhibit different disease phenotypes and may require additional clinical interventions alongside dietary modifications. Inadequate reporting of medication usage also contributed to the heterogeneity in the included studies.
Some researchers raised concerns about the potential risks following HPD, such as osteoporosis and kidney damage, however none of the studies included in our analysis reported any serious adverse events associated with HPDs, thus high-protein intake which provides around 30% of dietary calories or 1.5–2.0 g/kg/d is generally safe in women with PCOS. Theoretically, HPD may promote urinary calcium excretion resulting in calcium loss, but protein also increases intestinal calcium absorption and circulating insulin-like growth factor-I, while decreasing parathyroid hormone, which sufficiently counteracts the negative effects of protein acid loading on bone health. Therefore, systemic calcium homoeostasis and bone status were not negatively affected by the increased acid load associated with high protein [59], and the previous evidence did not identify a significantly unfavourable effect of protein intake on lumbar spine bone mineral density [60]. Due to the increased urinary calcium loss, nephrolithiasis is another risk of HPD intervention. However, with weight loss, the risk of metabolic syndrome-associated nephrolithiasis could be reduced [61]. Overall, it remains prudent to check with medical history and perform the necessary tests to assess the risk of nephrolithiasis before starting an HPD regimen.
Renal function is also an aspect that should be checked before HPD intervention because high protein consumption may increase glomerular hyperfiltration, but there is little evidence for such side effects within 24 months of HPD intervention in people without established renal disease [16, 62, 63]. In turn, weight loss reduces obesity-related kidney damage by decreasing renal filtration rate. However, HPD has the potential to cause further decline in renal function in patients with renal insufficiency and should be avoided [63, 64]. Because chronic kidney disease is often silent, patients should be screened for kidney function, such as serum creatinine and proteinuria, before the initiation of HP intervention. The upper limit of protein intake and intervention duration is not clearly defined, but based on included studies, basically, HPD up to 1.66 g/kg/day or accounts for 25–30% of the total energy intake for 4–16 weeks does not pose adverse effects.
To evaluate potential bias in favour of intervention adherence, we calculated the completion rates of the included studies. In the trials lasting 12 weeks or more [33,34,35,36, 38, 40], 91.6% of the patients completed the intervention, whereas in the trials lasting <12 weeks, 81% of the patients completed the intervention. No significant differences were observed between the intervention and control groups, indicating a high level of compliance with the dietary approach. However, the included studies did not describe specific information about the source and quality of protein, fats, and carbohydrates, especially the glycemic index, which are potential factors affecting metabolism. Moreover, exercise compliance may also have an impact on the results and should be rigorously standardised and reported, analysed as potential confounders in the future studies, in order to make the results more directly applicable to the clinical workplace.
To our knowledge, this is the first meta-analysis to summarise the effects of HPDs on women with PCOS. The strength of this study is that it included RCTs involving patients with PCOS undergoing high-protein interventions and relative subgroup analyses as a means of reducing study heterogeneity. However, some of the included studies did not report the SDs of change from baseline, which is one of the limitations. Therefore, the data were handled using Follman’s formula, and the results may have been affected using calculation. The second limitation is that the limited duration of intervention (4–16 weeks) in current studies, covering up the truly long-term effects of HPDs and calling for the requirement of further research focusing longer intervention. More importantly, when conducting subgroup analysis, we found that different intervention duration may be associated with different metabolic improvement, so future research could have a longer intervention duration, and monitor the metabolic changes at different time points in order to understand the metabolic improvement effects of different intervention duration. Furthermore, heterogeneity was found in many comparisons, rendering the difficulty in utility in clinical practice, even though we tried out best to minimize it by performing subgroup analysis. In addition, the number of people included in this meta-analysis is limited, with participants of each group in the studies ranging from 9–37. Thus, the multi-centre with larger sample size studies are needed in the further research in order to make the results more reliable. The final limitation is the ratio of carbohydrate in the control group was higher than that in intervention diet, therefore it is difficult to determine which nutrient affects the outcomes of interest. It is the fact that, high protein is along with low in carbohydrate when fat intake fixed in a food pattern, but we could do more basic research on the metabolic pathway to identify whether the improvement is contributed to high protein or low carbohydrate, or both.
Conclusion
Compared with isoenergetic BDs, HPDs are associated with more favourable improvements in FINS and HOMA-IR, whereas they have similar effects on body weight, abdominal obesity, lipid metabolism, and sex hormones. Therefore on the basis of limiting the total energy, HPDs could be adopted as nutritional intervention for PCOS management in clinical practice, especially for insulin resistance improvement, but it is significant to screen kidney function and risk of nephrolithiasis before HPD intervention. Due to the high heterogeneity, further research with consideration of different phenotypes of PCOS in larger and broader settings and longer intervention duration is required. In addition, the overall effect of HPDs and potential mechanism behind this should be further elucidated beyond the context of intermediate biomarkers and other crucial clinical outcomes, such as the risks of diabetes, cardiovascular disease, ovulatory cycle disturbances, and infertility in patients with PCOS.
References
Cassar S, Misso ML, Hopkins WG, Shaw CS, Teede HJ, Stepto NK. Insulin resistance in polycystic ovary syndrome: a systematic review and meta-analysis of euglycaemic-hyperinsulinaemic clamp studies. Hum Reprod. 2016;31:2619–31.
Lim SS, Davies MJ, Norman RJ, Moran LJ. Overweight, obesity and central obesity in women with polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. 2012;18:618–37.
Kakoly NS, Earnest A, Teede HJ, Moran LJ, Joham AE. The Impact of obesity on the incidence of type 2 diabetes among women with polycystic ovary syndrome. Diabetes Care. 2019;42:560–7.
Dumesic DA, Oberfield SE, Stener-Victorin E, Marshall JC, Laven JS, Legro RS. Scientific statement on the diagnostic criteria, epidemiology, pathophysiology and molecular genetics of polycystic ovary syndrome. Endocr Rev. 2015;36:487–525.
Cowan S, Lim S, Alycia C, Pirotta S, Thomson R, Gibson-Helm M, et al. Lifestyle management in polycystic ovary syndrome—beyond diet and physical activity. BMC Endocr Disord. 2023;23:14.
Dunaif A. Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis. Endocr Rev. 1997;18:774–800.
Glueck CJ, Goldenberg N. Characteristics of obesity in polycystic ovary syndrome: etiology, treatment and genetics. Metabolism. 2019;92:108–20.
Oberg E, Lundell C, Blomberg L, Gidlöf SB, Egnell PT, Hirschberg AL. Psychological well-being and personality in relation to weight loss following behavioral modification intervention in obese women with polycystic ovary syndrome: a randomized controlled trial. Eur J Endocrinol. 2020;183:1–11.
Goodman NF, Cobin RH, Futterweit W, Glueck JS, Legro RS, Carmina EGuide to the best practice in the evaluation and treatment of polycystic ovary syndrome - part 2. Endocr Pract. 2015;21:1415–26.
Teede HJ, Misso ML, Costello MF, Dokras A, Laven J, Moran L, et al. International PCOS Network. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Fertil Steril. 2018;110:364–79.
Lin AW, Lujan ME. Comparison of dietary intake and physical activity between women with and without polycystic ovary syndrome: a review. Adv Nutr. 2014;5:486–96.
Kazemi M, McBreairty LE, Chizen DR, Pierson RA, Chilibeck PD, Zello GA. A comparison of a pulse-based diet and the therapeutic lifestyle changes diet in combination with exercise and health counselling on the cardio-metabolic risk profile in Women with polycystic ovary syndrome: a randomized controlled trial. Nutrients. 2018;10:1387.
Teede HJ, Joham AE, Paul E, Moran LJ, Loxton D, Jolley D, et al. Longitudinal weight gain in women identified with polycystic ovary syndrome: results of an observational study in young women. Obesity (Silver Spring). 2013;21:1526–32.
Brutocao C, Zaiem F, Alsawas M, Morrow AS, Murad MH, Javed A. Psychiatric disorders in women with polycystic ovary syndrome: a systematic review and meta-analysis. Endocrine. 2018;62:318–25.
Moran LJ, Noakes M, Clifton PM, Wittert GA, Tomlinson L, Galletly C, et al. Ghrelin and measures of satiety are altered in polycystic ovary syndrome but not differentially affected by diet composition. J Clin Endocrinol Metab. 2004;89:3337–44.
Tang M, Armstrong CL, Leidy HJ, Campbell WW. Normal vs. high-protein weight loss diets in men: effects on body composition and indices of metabolic syndrome. Obesity (Silver Spring). 2013;21:E204–10.
Freire R. Scientific evidence of diets for weight loss: different macronutrient composition, intermittent fasting, and popular diets. Nutrition. 2020;69:110549.
Yu Z, Nan F, Wang LY, Jiang H, Chen W, Jiang Y. Effects of high-protein diet on glycemic control, insulin resistance and blood pressure in type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Clin Nutr. 2020;39:1724–34.
Barrea L, Arnone A, Annunziata G, Muscogiuri G, Laudisio D, Salzano C, et al. Adherence to the mediterranean diet, dietary patterns and body composition in women with polycystic ovary syndrome (PCOS). Nutrients. 2019;11:2278.
Baskin KK, Winders BR, Olson EN. Muscle as a “mediator“ of systemic metabolism. Cell Metab. 2015;21:237–48.
Abraham Gnanadass S, Divakar Prabhu Y, Valsala Gopalakrishnan A. Association of metabolic and inflammatory markers with polycystic ovarian syndrome (PCOS): an update. Arch Gynecol Obstet. 2021;303:631–43.
Li CW, Yu K, Shyh-Chang N, Li GX, Jiang LJ, Yu SL, et al. Circulating factors associated with sarcopenia during ageing and after intensive lifestyle intervention. J Cachexia Sarcopenia Muscle. 2019;10:586–600.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700.
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions, 6.0 ed. Cochrane; 2019. Available from: http://www.training.cochrane.org/handbook.
Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. 2018;27:1785–805.
Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13 https://doi.org/10.1186/1471-2288-5-13.
Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60.
Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–101.
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34.
Toscani MK, Mario FM, Radavelli-Bagatini S, Wiltgen D, Matos MC, Spritzer PM. Effect of high-protein or normal-protein diet on weight loss, body composition, hormone, and metabolic profile in southern Brazilian women with polycystic ovary syndrome: a randomized study. Gynecol Endocrinol. 2011;27:925–30.
Stamets K, Taylor DS, Kunselman A, Demers LM, Pelkman CL, Legro RS. A randomized trial of the effects of two types of short-term hypocaloric diets on weight loss in women with polycystic ovary syndrome. Fertil Steril. 2004;81:630–7.
Moran LJ, Noakes M, Clifton PM, Norman RJ. The effect of modifying dietary protein and carbohydrate in weight loss on arterial compliance and postprandial lipidemia in overweight women with polycystic ovary syndrome. Fertil Steril. 2010;94:2451–4.
Moran LJ, Noakes M, Clifton PM, Tomlinson L, Galletly C, Norman RJ. Dietary composition in restoring reproductive and metabolic physiology in overweight women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2003;88:812–9.
Mehrabani HH, Salehpour S, Amiri Z, Farahani SJ, Meyer BJ, Tahbaz F. Beneficial effects of a high-protein, low-glycemic-load hypocaloric diet in overweight and obese women with polycystic ovary syndrome: a randomized controlled intervention study. J Am Coll Nutr. 2012;31:117–25.
Elham HM, Akram G-A, Nahid R-J, Mohammad M, Nooshin A, Seyedeh MN, et al. Effect of fennel supplementation along with high-protein, low-carbohydrate weight-loss diet on insulin resistance and percentage of fat and muscle mass in overweight/obese women with polycystic ovary syndrome. J Funct Foods. 2020;67:103848.
Kasim-Karakas SE, Almario RU, Cunningham W. Effects of protein versus simple sugar intake on weight loss in polycystic ovary syndrome (according to the national institutes of health criteria. Fertil Steril. 2009;92:262–70.
Nadjarzadeh A, Ghadiri-Anari A, Ramezani-Jolfaie N, Mohammadi M, Salehi-Abargouei A, Namayande SM, et al. Effect of hypocaloric high-protein, low-carbohydrate diet supplemented with fennel on androgenic and anthropometric indices in overweight and obese women with polycystic ovary syndrome: a randomized placebo-controlled trial. Complement Ther Med. 2021;56:102633.
Dou P, Zhang TT, Xu Y, Xue Q, Shang J, Yang XL. [Effects of three medical nutrition therapies for weight loss on metabolic parameters and androgen level in overweight/obese patients with polycystic ovary syndrome. Zhonghua Yi Xue Za Zhi. 2023;103:1035–41.
Chen DM, Yang L, Jiang JC. The effects of high protein diet on glucolipid metabolism and BMI level in obese patients with polycystic ovary syndrome. Jiceng Yi Xue Lun Tan. 2021;25:3.
Baba NH, Sawaya S, Torbay N, Habbal Z, Azar S, Hashim SA. High protein vs high carbohydrate hypoenergetic diet for the treatment of obese hyperinsulinemic subjects. Int J Obes Relat Metab Disord. 1999;23:1202–6.
Farnsworth E, Luscombe ND, Noakes M, Wittert G, Argyiou E, Clifton PM. Effect of a high-protein, energy-restricted diet on body composition, glycemic control, and lipid concentrations in overweight and obese hyperinsulinemic men and women. Am J Clin Nutr. 2003;78:31–9.
Kazemi M, Hadi A, Pierson RA, Lujan ME, Zello GA, Chilibeck PD. Effects of dietary glycemic index and glycemic load on cardiometabolic and reproductive profiles in women with polycystic ovary syndrome: a systematic review and meta-analysis of randomized controlled trials. Adv Nutr. 2021;12:161–78.
Foster GD, Wyatt HR, Hill JO, McGuckin BG, Brill C, Mohammed BS, et al. A randomized trial of a low-carbohydrate diet for obesity. N Engl J Med. 2003;348:2082–90.
Leidy HJ, Carnell NS, Mattes RD, Campbell WW. Higher protein intake preserves lean mass and satiety with weight loss in pre-obese and obese women. Obesity (Silver Spring). 2007;15:421–9.
Luscombe ND, Clifton PM, Noakes M, Farnsworth E, Wittert G. Effect of a high-protein, energy-restricted diet on weight loss and energy expenditure after weight stabilization in hyperinsulinemic subjects. Int J Obes Relat Metab Disord. 2003;27:582–90.
Pesta DH, Samuel VT. A high-protein diet for reducing body fat: mechanisms and possible caveats. Nutr Metab (Lond). 2014;11:53.
Cuenca-Sánchez M, Navas-Carrillo D, Orenes-Piñero E. Controversies surrounding high-protein diet intake: satiating effect and kidney and bone health. Adv Nutr. 2015;6:260–6.
Davidenko O, Darcel N, Fromentin G, Tomé D. Control of protein and energy intake—brain mechanisms. Eur J Clin Nutr. 2013;67:455–61. https://doi.org/10.1038/ejcn.2013.73. May
Belza A, Ritz C, Sørensen MQ, Holst JJ, Rehfeld JF, Astrup A. Contribution of gastroenteropancreatic appetite hormones to protein-induced satiety. Am J Clin Nutr. 2013;97:980–9.
Journel M, Chaumontet C, Darcel N, Fromentin G, Tomé D. Brain responses to high-protein diets. Adv Nutr. 2012;3:322–9.
Layman DK, Shiue H, Sather C, Erickson DJ, Baum J. Increased dietary protein modifies glucose and insulin homeostasis in adult women during weight loss. J Nutr. 2003;133:405–10.
Nesti L, Mengozzi A, Tricò D. Impact of nutrient type and sequence on glucose tolerance: physiological insights and therapeutic implications. Front Endocrinol (Lausanne). 2019;10:144.
Tricò D, Frascerra S, Baldi S, Mengozzi A, Nesti L, Mari A, et al. The insulinotropic effect of a high-protein nutrient preload is mediated by the increase of plasma amino acids in type 2 diabetes. Eur J Nutr. 2019;58:2253–61.
Brinkworth GD, Noakes M, Keogh JB, Luscombe ND, Wittert GA, Clifton PM. Long-term effects of a high-protein, low-carbohydrate diet on weight control and cardiovascular risk markers in obese hyperinsulinemic subjects. Int J Obes Relat Metab Disord. 2004;28:661–70.
Sacks FM, Bray GA, Carey VJ, Smith SR, Ryan DH, Anton SD, et al. Comparison of weight-loss diets with different compositions of fat, protein and carbohydrates. N Engl J Med. 2009;360:859–73.
DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237:E214–23.
Diamanti-Kandarakis E, Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr Rev. 2012;33:981–1030.
Cao JJ, Nielsen FH. Acid diet (high-meat protein) effects on calcium metabolism and bone health. Curr Opin Clin Nutr Metab Care. 2010;13:698–702.
Darling AL, Millward DJ, Torgerson DJ, Hewitt CE, Lanham-New SA. Dietary protein and bone health: a systematic review and meta-analysis. Am J Clin Nutr. 2009;90:1674–92.
Rahman IA, Nusaly IF, Syahrir S, Nusaly H, Mansyur MA. Association between metabolic syndrome components and the risk of developing nephrolithiasis: a systematic review and bayesian meta-analysis. F1000Res. 2021;10:104.
Li Z, Treyzon L, Chen S, Yan E, Thames G, Carpenter CL. Protein-enriched meal replacements do not adversely affect liver, kidney or bone density: an outpatient randomized controlled trial. Nutr J. 2010;9:72.
Knight EL, Stampfer MJ, Hankinson SE, Spiegelman D, Curhan GC. The impact of protein intake on renal function decline in women with normal renal function or mild renal insufficiency. Ann Intern Med. 2003;138:460–7.
Cirillo M, Lombardi C, Chiricone D, De Santo NG, Zanchetti A, Bilancio G. Protein intake and kidney function in the middle-age population: contrast between cross-sectional and longitudinal data. Nephrol Dial Transplant. 2014;29:1733–40.
Acknowledgements
The authors thank Guannan Luan for helping with the literature search process. This manuscript was edited by Wallace Academic Editing.
Funding
This research was supported by National High Level Hospital Clinical Research Funding (no. 2022-PUMCH-B-055). The funding source had no role in the study design or in the execution, data collection or analysis, or manuscript writing.
Author information
Authors and Affiliations
Contributions
FW and PJL conceptualized the study and reviewed the manuscript for intellectual content. DP and WW collected the data, performed the statistical analysis. FW and PJL wrote the manuscript. PD contributed to data collection. PJL, FW, and PD revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, F., Dou, P., Wei, W. et al. Effects of high-protein diets on the cardiometabolic factors and reproductive hormones of women with polycystic ovary syndrome: a systematic review and meta-analysis. Nutr. Diabetes 14, 6 (2024). https://doi.org/10.1038/s41387-024-00263-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41387-024-00263-9