Abstract
Background
Gestational Diabetes Mellitus (GDM) is hyperglycaemia first detected during pregnancy. Globally, GDM affects around 1 in 6 live births (up to 1 in 4 in low- and middle-income countries- LMICs), thus, urgent measures are needed to prevent this public health threat.
Objective
To determine the effectiveness of pre-pregnancy lifestyle in preventing GDM.
Methods
We searched MEDLINE, Web of science, Embase and Cochrane central register of controlled trials. Randomized control trials (RCTs), case-control studies, and cohort studies that assessed the effect of pre-pregnancy lifestyle (diet and/or physical activity based) in preventing GDM were included. Random effects model was used to calculate odds ratio (OR) with 95% confidence interval. The Cochrane ROB-2 and the Newcastle-Ottawa Scale were used for assessing the risk of bias. The protocol was registered in PROSPERO (ID: CRD42020189574)
Results
Database search identified 7935 studies, of which 30 studies with 257,876 pregnancies were included. Meta-analysis of the RCTs (N = 5; n = 2471) in women who received pre-pregnancy lifestyle intervention showed non-significant reduction of the risk of developing GDM (OR 0.76, 95% CI: 0.50–1.17, p = 0.21). Meta-analysis of cohort studies showed that women who were physically active pre-pregnancy (N = 4; n = 23263), those who followed a low carbohydrate/low sugar diet (N = 4; n = 25739) and those women with higher quality diet scores were 29%, 14% and 28% less likely to develop GDM respectively (OR 0.71, 95% CI: 0.57, 0.88, p = 0.002, OR 0.86, 95% CI: 0.68, 1.09, p = 0.22 and OR 0.72, 95% CI 0.60–0.87, p = 0.0006).
Conclusion
This study highlights that some components of pre-pregnancy lifestyle interventions/exposures such as diet/physical activity-based preparation/counseling, intake of vegetables, fruits, low carbohydrate/low sugar diet, higher quality diet scores and high physical activity can reduce the risk of developing gestational diabetes. Evidence from RCTs globally and the number of studies in LMICs are limited, highlighting the need for carefully designed RCTs that combine the different aspects of the lifestyle and are personalized to achieve better clinical and cost effectiveness.
Similar content being viewed by others
Background
GDM is defined as the presence of hyperglycaemia, first detected any time during pregnancy [1]. Globally around, 20 million or 16% of all live births are affected by hyperglycaemia during pregnancy, of which more than 90% are present in LMICs [2]. Typically, GDM is diagnosed between 24 and 28 weeks of gestation [3]. However, varying degrees of hyperglycaemia may be present before this time period [4] and adverse effects on the fetus may have already happened at the time of diagnosis of GDM [5,6,7].
Maternal metabolic health during pregnancy has critical influence on the metabolic health of the offspring and possibly even in the subsequent generations [8]. There is increasing evidence that pre-conception health of women is of critical importance in shaping the metabolic health of the next generation [9,10,11]. In addition, GDM has been shown to be associated with adverse fetal programming [12]. Even in research setting, current management strategy for GDM, at best, reduces the short-term complications by about 50% [13, 14]. Thus, the focus needs to move away from “diagnosis and treatment of GDM” to “prevention of GDM.” Prevention of GDM may provide a crucial opportunity to reduce this risk of adverse metabolic programming of the offspring and future risk of cardiometabolic disorders, in addition to benefiting the mothers [15].
Lifestyle interventions are proven to effectively prevent type-2 diabetes [16]. Hence it is conceivable that such lifestyle interventions could prevent GDM. However, studies that tested the effectiveness of lifestyle interventions provided during pregnancy on GDM have shown mixed results [14, 17,18,19,20,21]. Interestingly, interventions provided during early weeks of gestation showed promising results [22]. Data from International Weight Management in Pregnancy Collaborative (iWIP) suggest that with careful selection of subjects and the type of intervention, GDM can be prevented if the interventions are carried out in early pregnancy [23]. A recent meta-analysis shows that antenatal structured diet and physical activity-based lifestyle interventions were linked to reduced gestational weight gain and lower risk of other adverse maternal and neonatal outcomes [23] and two individual participant data (IPD) meta-analysis on prevention of GDM are ongoing [24, 25]. However, this approach may still not reduce the adverse programming that may happen at or soon after the time of conception. Therefore, the best way to abolish this excess risk is by prevention of GDM with interventions before pregnancy.
Two recent studies have shown that lifestyle interventions before pregnancy may help to prevent GDM. However, these studies are small, of varying quality and used different components of “lifestyle interventions” [26, 27]. Not many have reported in LMICs, where the biggest burden of GDM is present. This systematic review aimed to summarize the available evidence from randomized control trials (RCTs), case-control studies and cohort studies for the various components of pre-pregnancy lifestyle in reducing the risk of GDM. Where possible, we conducted meta-analysis of the studies to quantify the effect of these components.
Methods
Search strategy
This is a systematic review and meta-analysis summarizing the evidence linking “pre-pregnancy” lifestyle (diet and/or physical activity aspects) and GDM risk and reporting the results of the analysis using a robust estimate, that is, Odds Ratio (OR). The PI(E)CO framework for this study is as follows: Population: Pregnant women, Intervention/Exposure: Pre-pregnancy diet and/or physical activity-based lifestyle intervention/exposure, Comparison: No pre-pregnancy diet and/or physical activity-based lifestyle intervention/exposure, Outcomes: Prevention of GDM/reduced risk of developing GDM.
The following electronic databases were searched: MEDLINE, Web of Science, Embase and Cochrane Central Register of Controlled Trials (CENTRAL). The search term combinations were designed with appropriate MeSH, free text and word variants to capture all studies on “Pre-pregnancy lifestyle interventions/components preventing Gestational Diabetes Mellitus.”
The search carried out were “[(Pregnant women OR Pregnancy OR Pre-pregnancy) AND (Lifestyle intervention OR Diet OR Exercise OR Behavioral change intervention OR Lifestyle education) AND (Gestational Diabetes OR Gestational Diabetes Mellitus OR Hyperglycaemia during Pregnancy)]”. All studies from inception till July 2022 were searched. All database searches were limited to “human” studies in order to eliminate animal model and other irrelevant studies. No Language restrictions were applied. The protocol was registered in PROSPERO (ID: CRD42020189574).
Study selection
Study inclusion
Studies that are randomized control trials (RCTs), cohort studies and case-control studies that assessed the effect of pre-pregnancy lifestyle (Diet and/or physical activity based) in preventing GDM were included.
Study exclusion
Studies involving women with type-1 or type-2 diabetes, women aged <18 and more than 50 years, women on metformin therapy (up to 6 weeks before) for anovulation and/or infertility, women with severe anaemia defined as hemoglobin (Hb) < 80 g/L, sickle cell traits, sickle cell anaemia and other genetic Hb variants and any other serious medical illness, any lifestyle intervention started only during pregnancy, drug interventions unless they had a separate lifestyle arm to prevent GDM, lifestyle intervention that have not reported incidence of GDM in their outcomes were excluded.
Two reviewers (SS and DP) independently performed the study selection. Rayyan, a web-based tool, was used to do the screening and selection. Any conflicts arising in study selection was resolved after a discussion. A third reviewer (NS) resolved any conflict arising in study selection or during quality assessment. Reference lists and gray literature were also searched to capture any unpublished data and additional studies. Following initial title and abstract screening, full-text screening was carried out.
Data extraction
Two independent authors extracted data of the selected studies. The data extracted were author, year, type of lifestyle intervention, time of lifestyle intervention/healthy lifestyle exposure, number of participants, ethnicity, country of study, mean BMI, mean age, Odds Ratio, Relative risk and other reported results. We extracted data on the number of events (GDM and non-GDM) in women exposed and not exposed to any lifestyle factors.
Risk of bias assessment
Two independent reviewers assessed the quality of the selected studies. All the following sources of bias were addressed: blinding, sequence generation, incomplete outcome data, allocation concealment, selective outcome reporting and others. The Cochrane ROB (Risk of Bias)-2 tool was used for assessing the quality of the selected randomized control trials. The Newcastle-Ottawa Scale (NOS) for assessing the quality of non-randomized studies. Based on the NOS, (0–9 for case-control studies / 0–10 for cohort studies) studies were categorized as follows: low risk (6–9/10), some concerns (3–5), high risk (0–2). The Publication bias and small studies effect quality assessment was carried out by assessing funnel plots using Egger’s tests.
Strategy for data synthesis
The outcome assessed is the risk of GDM. Random effects model using Mantel-Haenszel method was used to calculate odds ratio (OR) with 95% confidence interval from the relevant summary estimates reported by the included studies. Studies from the same study cohort are considered only once for meta-analysis but are listed in the summary table if any useful insight are provided based on components of intervention/exposure. For cohort studies that reported the events of GDM and non-GDM for various categories of diet and/or physical activity measures, studies with similar exposure group (low carb intake/low sugar diet group, higher quality diet scores, high physical activity group) were taken into consideration for meta-analysis. Cohort studies that report association of GDM and different components of diet and physical activity are included as separate studies for the purpose of this review. Statistical heterogeneity between the studies was assessed using I2 statistics. RevMan software version 5.4.1 was used for the statistical analysis.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
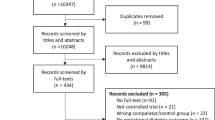
The initial databases search found 7935 studies and an additional 32 studies were identified from other sources (Fig. 1). Removing duplicates and abstract screening resulted in 510 studies for full-text screening and 30 were tabulated (Tables 1–4). Five of them were RCTs (n = 2471 pregnancies) [28,29,30,31,32], 4 case-control studies (n = 19,778 pregnancies) [33,34,35,36], and 21 cohort studies (n = 235,627 pregnancies). Of these, 5 were physical activity based (n = 46197 pregnancies) [27, 37,38,39,40], and 16 were diet based exploring various components (n = 189,430 pregnancies) [27, 41,42,43,44,45,46,47,48,49,50,51,52,53,54,55]. Full text of one of the RCTs was in Russian and hence only the data from the abstract was used [28]. The study characteristics based on the type and lifestyle components were summarized in Tables 1–4. Three RCTs tested a combination of diet and physical activity factors from high income countries. Two RCTs tested diet and were from low- and middle-income countries.
Meta-analysis were carried out to assess the pooled effect size by study type and components of lifestyle measures. Figure 2a shows the effect of pre-pregnancy RCTs of lifestyle intervention. 3 out of 5 of these studies were dietary interventions. Women in the intervention group were 24% less likely to develop GDM but this was not statistically significant (OR 0.76, 95% CI: 0.50–1.17, p = 0.21). Sub-group analysis of the studies from HICs and LMICs was also performed as shown in Fig. 2a. These studies had moderate heterogeneity. Quality assessment of these RCTs revealed that only 20% of the studies had low risk of bias. The bias was mainly due to randomization process and missing outcome data (Supplementary Fig. 1).
Three of the case-control studies studied dietary patterns and other case-control study focused on Dietary Inflammatory Index (DII). Meta-analysis of case-control studies was not performed as 2 studies are from the same study population and the dietary exposures are very heterogenous to combine in a meta-analysis. Quality assessment of the case-control studies revealed that low risk of bias with Newcastle-Ottawa scale score 8 (Supplementary Table 1)
Meta-analysis of cohort studies (n = 4) revealed that women who were more physically active before pregnancy were 34% less likely to develop GDM (OR 0.66, 95% CI: 0.44, 0.99, p = 0.04) (Fig. 2b). Similarly, women who had low carbohydrate/low sugar diet were 24% less likely to develop GDM (OR 0.86, 95% CI 0.68–1.09, p = 0.22) (Fig. 2c). Meta-analysis (Fig. 2d) of cohort studies linking diet score and GDM showed that women with higher quality diet scores were 28% less likely to develop GDM (OR 0.72, 95%CI: 0.60, 0.87, p = 0.0006). These studies had substantial heterogeneity among them. Quality assessment of the cohort studies revealed low risk of bias with Newcastle-Ottawa scale score 8 (Supplementary Table 1). 24 of all 30 studies were from high income countries and 6 were from low- and middle- income countries (Fig. 3). Supplementary Figs 2a-2d show the funnel plots for publication bias, which reveal symmetry and show very low risk of publications bias. Supplementary Figs 3a-3d show the sensitivity analysis based on leave-one out plots confirming the robustness of the results reported.
Discussion
Our systematic review and meta-analysis summarize crucial evidence highlighting the importance of “pre-pregnancy” lifestyle in reducing the risk of developing GDM. To our knowledge, our systematic review and meta-analysis is the first to show the evidence for pre-pregnancy lifestyle interventions and the risk of GDM. Data from RCTs were limited and showed that pre-pregnancy lifestyle interventions may reduce the risk of developing GDM by about 24%. The cohort studies explored various components of diet and physical activity measures and confirmed a strong link between higher self-reported physical activity and diets that are classified as “healthy” during the pre-pregnancy period and reduced risk of GDM. Analysis of cohort studies on low carbohydrate/low sugar diet and higher quality diet scores also showed positive effect in reducing the risk of developing GDM.
A recent systematic review during pregnancy showed that structured dietary intervention can reduce the risk of GDM by about 39% in 3029 women [23] The findings from our RCTs however did not show a significant reduction in the risk of GDM in women who received lifestyle intervention before pregnancy. This may be due to the heterogeneity of the studies using different components of the lifestyle intervention and due to the smaller number of pre-pregnancy trials conducted thus far. Nevertheless, it provides crucial evidence that lifestyle intervention before pregnancy is doable. Additionally, well-designed RCTs are required to assess the benefits of different components of dietary intervention. Interventions that focussed on physical activity during early pregnancy have been shown to reduce the development of GDM [23, 27] and type 2 diabetes in at risk individuals [16, 56]. While we did not find any RCTs that focussed only on physical activity alone interventions before pregnancy, the cohort studies summarize the available evidence and highlight the need for RCTs.
Strengths and limitations
Our study used an extensive search strategy and a robust methodology to include and evaluate the different components of dietary and physical activity interventions in the pre-pregnancy period. Inclusion of non-RCTs studies, provided a comprehensive summary and highlighted the need for future RCTs on different components of lifestyle interventions before pregnancy to reduce the risk of GDM. It also highlights the paucity of evidence from LMICs where the burden of GDM is very high. Our study however has the following limitations. Firstly, there is high heterogeneity between the studies. This is likely be due to the various different lifestyle factors that are included in these studies. Secondly, most of these cohort and case-control studies were based on food frequency and physical activity questionnaires, which would result in self-reported bias. This would need to be kept in mind and future studies should ideally use objective measures to minimize this bias. Finally, not many studies were conducted from LMICs and therefore our findings were not applicable to populations who have the highest risk of GDM.
Implications for future research
Our review also highlights the paucity of data from LMICs, which may highlight the potential difficulties of conducting RCTs, especially in the pre-pregnancy period. However, high birth rate in LMICs provide other windows of opportunities such as “inter-pregnancy” interval [26]. This may also provide opportunities for targeting women at high-risk, for example those who had GDM in their previous pregnancy, which may be more cost effective.
While our review highlights the potential benefits of various components of diet and lifestyle measures, studies that focus on reducing sedentary behavior are needed. Sedentary behavior is associated with higher metabolic and cardiovascular risk [57, 58] and break in sedentary behavior has been shown to improve glucose and metabolic profile in women post-menopause [59]. This approach may work during inter-pregnancy interventions, especially in women with previous history of GDM with young children.
Finally, future interventions should be co-developed and “personalized.” It is known that women from high-risk ethnic groups have difficulty in following “generalized” lifestyle intervention [60, 61]. Thus, it is critically important that these women receive a “personalized” lifestyle intervention that relates closely to their lifestyle. Newer technologies such as mobile phone based remote interventions offer promise that these can be achieved at a lower cost [62, 63]. Face-to-face physical activity interventions can be challenging in women with young children [64] and may increase the travel cost and time burden in LMICs. In LMICs, mobile phone connections are increasing and more accessible than clean water [65]. Thus, using mobile phone technology to deliver these interventions would be pragmatic and urgently needed.
Data availability
The data extracted and analyzed in this systematic review and meta-analysis are available from the corresponding author upon request.
References
WHO. Diagnostic Criteria and Classification of Hyperglycaemia First Detected in Pregnancy. 2013. https://apps.who.int/iris/bitstream/handle/10665/85975/WHO_NMH_MND_13.2_eng.pdf;jsessionid=E5806DC69967B1753795EB66C155EF30?sequence=1 (accessed 05.12. 2022).
International Diabetes Federation. IDF Diabetes Atlas teB, Belgium. IDF Diabetes Atlas, 10th edn. 2021. Available at: https://www.diabetesatlas.org (accessed 15.02. 2023).
Carpenter MW, Canick JA, Hogan JW, Shellum C, Somers M, Star JA. Amniotic fluid insulin at 14-20 weeks’ gestation: association with later maternal glucose intolerance and birth macrosomia. Diabetes Care. 2001;24:1259–63.
Riskin-Mashiah S, Younes G, Damti A, Auslender R. First-trimester fasting hyperglycemia and adverse pregnancy outcomes. Diabetes Care. 2009;32:1639–43.
Venkataraman H, Ram U, Craik S, Arungunasekaran A, Seshadri S, Saravanan P. Increased fetal adiposity prior to diagnosis of gestational diabetes in South Asians: more evidence for the ‘thin–fat’ baby. Diabetologia. 2017;60:399–405.
Ram U, Seshadri S, Saravanan P. Hyperglycaemia in pregnancy: time to ask the hard questions? Lancet Diabetes Endocrinol. 2017;5:578–9.
Sovio U, Murphy HR, Smith GC. Accelerated fetal growth prior to diagnosis of gestational diabetes mellitus: a prospective cohort study of nulliparous women. Diabetes Care. 2016;39:982–7.
Kwon EJ, Kim YJ. What is fetal programming?: A lifetime health is under the control of in utero health. Obstet Gynecol Sci. 2017;60:506–19.
Fleming TP, Watkins AJ, Velazquez MA, Mathers JC, Prentice AM, Stephenson J, et al. Origins of lifetime health around the time of conception: causes and consequences. Lancet. 2018;391:1842–52.
Barker M, Dombrowski SU, Colbourn T, Fall C, Kriznik NM, Lawrence WT, et al. Intervention strategies to improve nutrition and health behaviours before conception. Lancet. 2018;391:1853–64.
Stephenson J, Heslehurst N, Hall J, Schoenaker D, Hutchinson J, Cade JE, et al. Before the beginning: nutrition and lifestyle in the preconception period and its importance for future health. Lancet. 2018;391:1830–41.
Monteiro LJ, Norman JE, Rice GE, Illanes SE. Fetal programming and gestational diabetes mellitus. Placenta. 2016;48:S54–s60.
Landon MB, Spong CY, Thom E, Carpenter MW, Ramin SM, Casey B, et al. A multicenter, randomized trial of treatment for mild gestational diabetes. N. Engl J Med. 2009;361:1339–48.
Bain E, Crane M, Tieu J, Han S, Crowther CA, Middleton P Diet and exercise interventions for preventing gestational diabetes mellitus. Cochrane Database Syst Rev. 2015;(4):Cd010443.
Saravanan P. Gestational diabetes: opportunities for improving maternal and child health. Lancet Diabetes Endocrinol. 2020;8:793–800.
Tuomilehto J, Lindström J, Eriksson JG, Valle TT, Hämäläinen H, Ilanne-Parikka P, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N. Engl J Med. 2001;344:1343–50.
Simmons D, Jelsma JG, Galjaard S, Devlieger R, van Assche A, Jans G, et al. Results from a european multicenter randomized trial of physical activity and/or healthy eating to reduce the risk of gestational diabetes mellitus: The DALI lifestyle pilot. Diabetes Care. 2015;38:1650–6.
Assaf-Balut C, García de la Torre N, Durán A, Fuentes M, Bordiú E, Del Valle L, et al. A Mediterranean diet with additional extra virgin olive oil and pistachios reduces the incidence of gestational diabetes mellitus (GDM): A randomized controlled trial: The St. Carlos GDM prevention study. PLoS One. 2017;12:e0185873.
Dodd JM, Turnbull D, McPhee AJ, Deussen AR, Grivell RM, Yelland LN, et al. Antenatal lifestyle advice for women who are overweight or obese: LIMIT randomised trial. BMJ: Br Med J. 2014;348:g1285.
Poston L, Bell R, Croker H, Flynn AC, Godfrey KM, Goff L, et al. Effect of a behavioural intervention in obese pregnant women (the UPBEAT study): a multicentre, randomised controlled trial. Lancet Diabetes Endocrinol. 2015;3:767–77.
Oteng-Ntim E, Varma R, Croker H, Poston L, Doyle P. Lifestyle interventions for overweight and obese pregnant women to improve pregnancy outcome: systematic review and meta-analysis. BMC Med. 2012;10:47.
Song C, Li J, Leng J, Ma RC, Yang X. Lifestyle intervention can reduce the risk of gestational diabetes: a meta-analysis of randomized controlled trials. Obes Rev. 2016;17:960–9.
Teede HJ, Bailey C, Moran LJ, Bahri Khomami M, Enticott J, Ranasinha S, et al. Association of Antenatal Diet and Physical Activity–Based Interventions With Gestational Weight Gain and Pregnancy Outcomes: A Systematic Review and Meta-analysis. JAMA Intern Med. 2022;182:106–14.
Boath A, Vale L, Hayes L, Allotey J, Heslehurst N. Differential effects of diet and physical activity interventions in pregnancy to prevent gestational diabetes mellitus and reduce gestational weight gain by level of maternal adiposity: a protocol for an individual patient data (IPD) meta-analysis of randomised controlled trials. BMJ Open. 2023;13:e065335.
Coomar D, Hazlehurst JM, Austin F, Foster C, Hitman GA, Heslehurst N, et al. Diet and physical activity in pregnancy to prevent gestational diabetes: a protocol for an individual participant data (IPD) meta-analysis on the differential effects of interventions with economic evaluation. BMJ Open. 2021;11:e048119.
Phelan S. Windows of opportunity for lifestyle interventions to prevent gestational diabetes mellitus. Am J Perinatol. 2016;33:1291–9.
Zhang C, Tobias DK, Chavarro JE, Bao W, Wang D, Ley SH, et al. Adherence to healthy lifestyle and risk of gestational diabetes mellitus: prospective cohort study. BMJ: Br Med J. 2014;349:g5450.
Makarova EL OAA, Terekhina NA Role of a comprehensive pregravid preparation program for obese women in the prevention of gestational complications. Akusherstvo i Ginekologiya. 2020;4:182–8.
Rönö K, Stach-Lempinen B, Eriksson JG, Pöyhönen-Alho M, Klemetti MM, Roine RP, et al. Prevention of gestational diabetes with a prepregnancy lifestyle intervention - findings from a randomized controlled trial. Int J Women’s Health. 2018;10:493–501.
Sahariah SA, Potdar RD, Gandhi M, Kehoe SH, Brown N, Sane H, et al. A daily snack containing leafy green vegetables, fruit, and milk before and during pregnancy prevents gestational diabetes in a randomized, controlled trial in Mumbai, India. J Nutr. 2016;146:1453s–60s.
Sun L, Niu Z A mushroom diet reduced the risk of pregnancy-induced hypertension and macrosomia: a randomized clinical trial. Food Nutr Res. 2020;64:4451.
Valkama AJ, Meinilä J, Koivusalo S, Lindström J, Rönö K, Stach-Lempinen B, et al. The effect of pre-pregnancy lifestyle counselling on food intakes and association between food intakes and gestational diabetes in high-risk women: results from a randomised controlled trial. J Hum Nutr Diet. 2018;31:301–5.
Asadi M, Shahzeidi M, Nadjarzadeh A, Hashemi Yusefabad H, Mansoori A. The relationship between pre-pregnancy dietary patterns adherence and risk of gestational diabetes mellitus in Iran: A case–control study. Nutr Dietetics. 2019;76:597–603.
Chen Q, Wu W, Yang H, Zhang P, Feng Y, Wang K, et al. A vegetable dietary pattern is associated with lowered risk of gestational diabetes mellitus in chinese women. Diabetes Metab J. 2020;44:887–96.
Chen Q, Feng Y, Yang H, Wu W, Zhang P, Wang K, et al. A vitamin pattern diet is associated with decreased risk of gestational diabetes mellitus in chinese women: results from a case control study in Taiyuan, China. J Diabetes Res. 2019;2019:5232308.
Shivappa N, Hébert JR, Akhoundan M, Mirmiran P, Rashidkhani B. Association between inflammatory potential of diet and odds of gestational diabetes mellitus among Iranian women. J Matern Fetal Neonatal Med. 2019;32:3552–8.
Currie LM, Woolcott CG, Fell DB, Armson BA, Dodds L. The association between physical activity and maternal and neonatal outcomes: a prospective cohort. Matern Child Health J. 2014;18:1823–30.
Dempsey JC, Sorensen TK, Williams MA, Lee IM, Miller RS, Dashow EE, et al. Prospective study of gestational diabetes mellitus risk in relation to maternal recreational physical activity before and during pregnancy. Am J Epidemiol. 2004;159:663–70.
Oken E, Ning Y, Rifas-Shiman SL, Radesky JS, Rich-Edwards JW, Gillman MW. Associations of physical activity and inactivity before and during pregnancy with glucose tolerance. Obstet Gynecol. 2006;108:1200–7.
Zhang C, Solomon CG, Manson JE, Hu FB. A prospective study of pregravid physical activity and sedentary behaviors in relation to the risk for gestational diabetes mellitus. Arch Intern Med. 2006;166:543–8.
Marí-Sanchis A, Díaz-Jurado G, Basterra-Gortari FJ, de la Fuente-Arrillaga C, Martínez-González MA, Bes-Rastrollo M. Association between pre-pregnancy consumption of meat, iron intake, and the risk of gestational diabetes: the SUN project. Eur J Nutr. 2018;57:939–49.
Bao W, Tobias DK, Olsen SF, Zhang C. Pre-pregnancy fried food consumption and the risk of gestational diabetes mellitus: a prospective cohort study. Diabetologia. 2014;57:2485–91.
Bao W, Song Y, Bertrand KA, Tobias DK, Olsen SF, Chavarro JE, et al. Prepregnancy habitual intake of vitamin D from diet and supplements in relation to risk of gestational diabetes mellitus: A prospective cohort study. J Diabetes. 2018;10:373–9.
Bao W, Bowers K, Tobias DK, Olsen SF, Chavarro J, Vaag A, et al. Prepregnancy low-carbohydrate dietary pattern and risk of gestational diabetes mellitus: a prospective cohort study. Am J Clin Nutr. 2014;99:1378–84.
Chen L, Hu FB, Yeung E, Willett W, Zhang C. Prospective study of pre-gravid sugar-sweetened beverage consumption and the risk of gestational diabetes mellitus. Diabetes Care. 2009;32:2236–41.
Li M, Li S, Chavarro JE, Gaskins AJ, Ley SH, Hinkle SN, et al. Prepregnancy habitual intakes of total, supplemental, and food folate and risk of gestational diabetes mellitus: A prospective cohort study. Diabetes Care. 2019;42:1034–41.
Looman M, Schoenaker D, Soedamah-Muthu SS, Geelen A, Feskens EJM, Mishra GD. Pre-pregnancy dietary carbohydrate quantity and quality, and risk of developing gestational diabetes: the Australian Longitudinal Study on Women’s Health. Br J Nutr. 2018;120:435–44.
Yee LM, Silver RM, Haas DM, Parry S, Mercer BM, Iams J, et al. Quality of periconceptional dietary intake and maternal and neonatal outcomes. Am J Obstet Gynecol. 2020;223:121.e1–e8.
Donazar-Ezcurra M, Lopez-Del Burgo C, Martinez-Gonzalez MA, Basterra-Gortari FJ, de Irala J, Bes-Rastrollo M. Pre-pregnancy adherences to empirically derived dietary patterns and gestational diabetes risk in a Mediterranean cohort: the Seguimiento Universidad de Navarra (SUN) project. Br J Nutr. 2017;118:715–21.
Donazar-Ezcurra M, Lopez-Del Burgo C, Martinez-Gonzalez MA, Basterra-Gortari FJ, de Irala J, Bes-Rastrollo M. Soft drink consumption and gestational diabetes risk in the SUN project. Clin Nutr. 2018;37:638–45.
Donazar-Ezcurra M, Lopez-Del Burgo C, Martinez-Gonzalez MA, Dominguez LJ, Basterra-Gortari FJ, de Irala J, et al. Association of the Dietary-Based Diabetes-Risk Score (DDS) with the risk of gestational diabetes mellitus in the Seguimiento Universidad de Navarra (SUN) project. Br J Nutr. 2019;122:800–7.
Schoenaker DA, Soedamah-Muthu SS, Callaway LK, Mishra GD. Pre-pregnancy dietary patterns and risk of gestational diabetes mellitus: results from an Australian population-based prospective cohort study. Diabetologia. 2015;58:2726–35.
Tobias DK, Zhang C, Chavarro J, Bowers K, Rich-Edwards J, Rosner B, et al. Prepregnancy adherence to dietary patterns and lower risk of gestational diabetes mellitus. Am J Clin Nutr. 2012;96:289–95.
Zhang C, Schulze MB, Solomon CG, Hu FB. A prospective study of dietary patterns, meat intake and the risk of gestational diabetes mellitus. Diabetologia. 2006;49:2604–13.
Zhang C, Liu S, Solomon CG, Hu FB. Dietary fiber intake, dietary glycemic load, and the risk for gestational diabetes mellitus. Diabetes Care. 2006;29:2223–30.
Group DPPDR. The Diabetes Prevention Program (DPP): description of lifestyle intervention. Diabetes Care. 2002;25:2165–71.
Chomistek AK, Manson JE, Stefanick ML, Lu B, Sands-Lincoln M, Going SB, et al. Relationship of sedentary behavior and physical activity to incident cardiovascular disease: results from the women’s health initiative. J Am Coll Cardiol. 2013;61:2346–54.
Edwardson CL, Gorely T, Davies MJ, Gray LJ, Khunti K, Wilmot EG, et al. Association of sedentary behaviour with metabolic syndrome: a meta-analysis. PLOS ONE. 2012;7:e34916.
Henson J, Davies MJ, Bodicoat DH, Edwardson CL, Gill JM, Stensel DJ, et al. Breaking up prolonged sitting with standing or walking attenuates the postprandial metabolic response in postmenopausal women: a randomized acute study. Diabetes Care. 2015;39:130–8.
Egan AM, Simmons D. Lessons learned from lifestyle prevention trials in gestational diabetes mellitus. Diabet Med. 2019;36:142–50.
Nicklas JM, Zera CA, Seely EW, Abdul-Rahim ZS, Rudloff ND, Levkoff SE. Identifying postpartum intervention approaches to prevent type 2 diabetes in women with a history of gestational diabetes. BMC Pregnancy Childbirth. 2011;11:23.
Sampathkumar S, Sankar M, Ramasamy S, Sriram N, Saravanan P, Ram U Uptake, Engagement and Acceptance, Barriers and Facilitators of a Text Messaging Intervention for Postnatal Care of Mother and Child in India—A Mixed Methods Feasibility Study. Int J Environ Res Public Health. 2022;19:8914.
Yadav P, Kant R, Kishore S, Barnwal S, Khapre. M The impact of mobile health interventions on antenatal and postnatal care utilization in low-and middle-income countries: a meta-analysis. Cureus. 2022;14:e21256.
Ekezie W, Dallosso H, Saravanan P, Khunti K, Hadjiconstantinou M. Experiences of using a digital type 2 diabetes prevention application designed to support women with previous gestational diabetes. BMC Health Serv Res. 2021;21:772.
McCool J, Dobson R, Whittaker R, Paton C. Mobile Health (mHealth) in low- and middle-income countries. Annu Rev Public Health. 2022;43:525–39.
Acknowledgements
Authors would like to thank the academic support librarian Samantha Johnson, University of Warwick for her support with database search. PS and YW are part supported by Medical Research Council, UK Grant number: MR/R020981/1. SS is funded by a scholarship from International Doctoral Training Program, supported by Novo Nordisk Plc, Copenhagen, Denmark and University of Warwick.
Author information
Authors and Affiliations
Contributions
SS, NS and PS designed the study and developed the protocol. SS and DP performed the literature search, study selection, and data extraction with the guidance of NS. SS and YW performed the statistical analysis. SS designed the tables, figures, and supplementary file, with the help of PS. SS and PS prepared the initial drafts of the manuscript, with inputs from NS, YW and DP. All authors contributed to the drafts and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sampathkumar, S., Parkhi, D., Ghebremichael-Weldeselassie, Y. et al. Effectiveness of pre-pregnancy lifestyle in preventing gestational diabetes mellitus—a systematic review and meta-analysis of 257,876 pregnancies. Nutr. Diabetes 13, 22 (2023). https://doi.org/10.1038/s41387-023-00251-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41387-023-00251-5