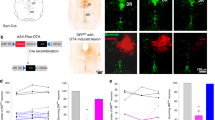
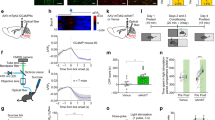
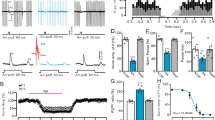
Abstract
In the central nervous system, noradrenaline transmission controls the degree to which we are awake, alert, and attentive. Aberrant noradrenaline transmission is associated with pathological forms of hyper- and hypo-arousal that present in numerous neuropsychiatric disorders often associated with dysfunction in serotonin transmission. In vivo, noradrenaline regulates the release of serotonin because noradrenergic input drives the serotonin neurons to fire action potentials via activation of excitatory α1-adrenergic receptors (α1-AR). Despite the critical influence of noradrenaline on the activity of dorsal raphe serotonin neurons, the source of noradrenergic afferents has not been resolved and the presynaptic mechanisms that regulate noradrenaline-dependent synaptic transmission have not been described. Using an acute brain slice preparation from male and female mice and electrophysiological recordings from dorsal raphe serotonin neurons, we found that selective optogenetic activation of locus coeruleus terminals in the dorsal raphe was sufficient to produce an α1-AR-mediated excitatory postsynaptic current (α1-AR-EPSC). Activation of inhibitory α2-adrenergic receptors (α2-AR) with UK-14,304 eliminated the α1-AR-EPSC via presynaptic inhibition of noradrenaline release, likely via inhibition of voltage-gated calcium channels. In a subset of serotonin neurons, activation of postsynaptic α2-AR produced an outward current through activation of GIRK potassium conductance. Further, in vivo activation of α2-AR by systemic administration of clonidine reduced the expression of c-fos in the dorsal raphe serotonin neurons, indicating reduced neural activity. Thus, α2-AR are critical regulators of serotonin neuron excitability.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 13 print issues and online access
$259.00 per year
only $19.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Aston-Jones G, Rajkowski J, Cohen J. Role of locus coeruleus in attention and behavioral flexibility. Biol Psychiatry. 1999;46:1309–20.
Agren H, Koulu M, Saavedra JM, Potter WZ, Linnoila M. Circadian covariation of norepinephrine and serotonin in the locus coeruleus and dorsal raphe nucleus in the rat. Brain Res. 1986;397:353–8.
Baraban JM, Wang RY, Aghajanian G. Reserpine suppression of dorsal raphe neuronal firing: mediation by adrenergic system. Eur J Pharmacol. 1978;52:27–36.
Baraban JM, Aghajanian GK. Suppression of serotonergic neuronal firing by alpha-adrenoceptor antagonists: evidence against GABA mediation. Eur J Pharmacol. 1980;66:287–94.
Baraban JM, Aghajanian GK. Suppression of firing activity of 5-HT neurons in the dorsal raphe by alpha-adrenoceptor antagonists. Neuropharmacology. 1980;19:355–63.
Pudovkina OL, Cremers TIFH, Westerink BHC. Regulation of the release of serotonin in the dorsal raphe nucleus by alpha1 and alpha2 adrenoceptors. Synapse. 2003;50:77–82.
Pudovkina OL, Cremers TIFH, Westerink BHC. The interaction between the locus coeruleus and dorsal raphe nucleus studied with dual-probe microdialysis. Eur J Pharmacol. 2002;445:37–42.
Svensson TH, Bunney BS, Aghajanian GK. Inhibition of both noradrenergic and serotonergic neurons in brain by the alpha-adrenergic agonist clonidine. Brain Res. 1975;92:291–306.
Bortolozzi A, Artigas F. Control of 5-hydroxytryptamine release in the dorsal raphe nucleus by the noradrenergic system in rat brain. Role of α-Adrenoceptors. Neuropsychopharmacology. 2002;28:421–34.
Vandermaelen CP, Aghajanian GK. Electrophysiological and pharmacological characterization of serotonergic dorsal raphe neurons recorded extracellularly and intracellularly in rat brain slices. Brain Res. 1983;289:109–19.
Gantz SC, Moussawi K, Hake HS. Delta glutamate receptor conductance drives excitation of mouse dorsal raphe neurons. Elife. 2020;9:103.
Khamma JK, Copeland DS, Hake HS, Gantz SC. Spatiotemporal control of noradrenaline-dependent synaptic transmission in mouse dorsal raphe serotonin neurons. J Neurosci. 2022;42:968–79.
Sheng M, Greenberg ME. The regulation and function of c-fos and other immediate early genes in the nervous system. Neuron. 1990;4:477–85.
Lindeberg J, Usoskin D, Bengtsson H, Gustafsson A, Kylberg A, Söderström S, et al. Transgenic expression of Cre recombinase from the tyrosine hydroxylase locus. Genesis. 2004;40:67–73.
Copeland DS, Gugel A, Gantz SC. Potentiation of neuronal activity by tonic GluD1 current in brain slices. EMBO Rep. 2023;24:e56801.
Saleeba C, Dempsey B, Le S, Goodchild A, McMullan S. A student’s guide to neural circuit tracing. Front Neurosci. 2019;13:897.
Grzanna R, Molliver ME. The locus coeruleus in the rat: an immunohistochemical delineation. NSC. 1980;5:21–40.
Robertson SD, Plummer NW, Marchena J, de, Jensen P. Developmental origins of central norepinephrine neuron diversity. Nature Publishing Group. 2013;16:1016–23.
Franklin KBJ, Paxinos G The mouse brain atlas in stereotaxic coordinates. 3rd edition, Elsevier: New York, NY; 2007.
Delfs JM, Zhu Y, Druhan JP, Aston-Jones GS. Origin of noradrenergic afferents to the shell subregion of the nucleus accumbens: anterograde and retrograde tract-tracing studies in the rat. Brain Res. 1998;806:127–40.
Ganley RP, Werder K, Wildner H, Zeilhofer HU. Spinally projecting noradrenergic neurons of the locus coeruleus display resistance to AAV2retro-mediated transduction. Mol Pain. 2021;17:17448069211037888.
Williams JT, Henderson G, North RA. Characterization of alpha 2-adrenoceptors which increase potassium conductance in rat locus coeruleus neurones. NSC. 1985;14:95–101.
Williams JT, Marshall KC. Membrane properties and adrenergic responses in locus coeruleus neurons of young rats. J Neurosci. 1987;7:3687–94.
Courtney NA, Ford CP. The timing of dopamine- and noradrenaline-mediated transmission reflects underlying differences in the extent of spillover and pooling. J Neurosci. 2014;34:7645–56.
Mateo Y, Pineda J, Meana JJ. Somatodendritic alpha2-adrenoceptors in the locus coeruleus are involved in the in vivo modulation of cortical noradrenaline release by the antidepressant desipramine. J Neurochem. 1998;71:790–8.
Washburn M, Moises HC. Electrophysiological correlates of presynaptic alpha 2-receptor-mediated inhibition of norepinephrine release at locus coeruleus synapses in dentate gyrus. J Neurosci. 1989;9:2131–40.
Wang B, Wang Y, Wu Q, Huang H, Li S. Effects of α2A adrenoceptors on norepinephrine secretion from the locus coeruleus during chronic stress-induced depression. Front Neurosci. 2017;11:1–8.
Trendelenburg A-U, Klebroff W, Hein L, Starke K. A study of presynaptic α2-autoreceptors in α2A/D-, α2B- and α2C-adrenoceptor-deficient mice. Naunyn-Schmiedeberg’s Arch Pharmacol. 2001;364:117–30.
Veldhuizen MJA, Feenstra MGP, Heinsbroek RPW, Boer GJ. In vivo microdialysis of noradrenaline overflow: effects of α‐adrenoceptor agonists and antagonists measured by cumulative concentration‐response curves. Br J Pharmacol. 1993;109:655–60.
L’Heureux R, Dennis T, Curet O, Scatton B. Measurement of endogenous noradrenaline release in the rat cerebral cortex in vivo by transcortical dialysis: effects of drugs affecting noradrenergic transmission. J Neurochem. 1986;46:1794–801.
Bücheler MM, Hadamek K, Hein L. Two α2-adrenergic receptor subtypes, α2A and α2C, inhibit transmitter release in the brain of gene-targeted mice. Neuroscience. 2002;109:819–26.
Frankhuijzen AL, Wardeh G, Hogenboom F, Mulder AH. Alpha 2-adrenoceptor mediated inhibition of the release of radiolabelled 5-hydroxytryptamine and noradrenaline from slices of the dorsal region of the rat brain. Naunyn Schmiedebergs Arch Pharmacol. 1988;337:255–60.
Ladera C, Godino MDC, Cabañero MJ, Torres M, Watanabe M, Luján R, et al. Pre‐synaptic GABAB receptors inhibit glutamate release through GIRK channels in rat cerebral cortex. J Neurochem. 2008;107:1506–17.
Llamosas N, Ugedo L, Torrecilla M. Inactivation of GIRK channels weakens the pre‐ and postsynaptic inhibitory activity in dorsal raphe neurons. Physiol Rep. 2017;5:e13141.
Michaeli A, Yaka R. Dopamine inhibits GABAA currents in ventral tegmental area dopamine neurons via activation of presynaptic G-protein coupled inwardly-rectifying potassium channels. Neuroscience. 2010;165:1159–69.
Lüscher C, Jan LY, Stoffel M, Malenka RC, Nicoll RA. G protein-coupled inwardly rectifying K+ channels (GIRKs) mediate postsynaptic but not presynaptic transmitter actions in Hippocampal neurons. Neuron. 1997;19:687–95.
Liu Z-L, Ma H, Xu R-X, Dai Y-W, Zhang H-T, Yao X-Q, et al. Potassium channels underlie postsynaptic but not presynaptic GABAB receptor-mediated inhibition on ventrolateral periaqueductal gray neurons. Brain Res Bull. 2012;88:529–33.
Sabater VG, Rigby M, Burrone J. Voltage-gated potassium channels ensure action potential shape fidelity in distal axons. J Neurosci. 2021;41:5372–85.
Bean BP. The action potential in mammalian central neurons. Nat Rev Neurosci. 2007;8:451–65.
Couch JR. Responses of neurons in the raphe nuclei to serotonin, norepinephrine and acetylcholine and their correlation with an excitatory synaptic input. Brain Res. 1970;19:137–50.
Yoshimura M, Higashi H, Nishi S. Noradrenaline mediates slow excitatory synaptic potentials in rat dorsal raphe neurons in vitro. Neurosci Lett. 1985;61:305–10.
Gantz SC, Levitt ES, Llamosas N, Neve KA, Williams JT. Depression of serotonin synaptic transmission by the dopamine precursor L-DOPA. Cell Reports. 2015;12:944–54.
Gantz SC, Bunzow JR, Williams JT. Spontaneous inhibitory synaptic currents mediated by a G protein-coupled receptor. Neuron. 2013;78:807–12.
Gantz SC, Ortiz MM, Belilos AJ, Moussawi K. Excitation of medium spiny neurons by “inhibitory” ultrapotent chemogenetics via shifts in chloride reversal potential. Elife. 2021;10:1–16.
Baraban JM, Aghajanian GK. Noradrenergic innervation of serotonergic neurons in the dorsal raphe: demonstration by electron microscopic autoradiography. Brain Res. 1981;204:1–11.
Loizou LA. Projections of the nucleus locus coeruleus in the albino rat. Brain Res. 1969;15:563–6.
Chu N-S, Bloom FE. The catecholamine-containing neurons in the cat dorsolateral pontine tegmentum: Distribution of the cell bodies and some axonal projections. Brain Res. 1974;66:1–21.
Roizen MF, Jacobowitz DM. Studies on the origin of innervation of the noradrenergic area bordering on the nucleus raphe dorsalis. Brain Res. 2002;101:561–8.
Bortel A Nature of DSP-4 induced neurotoxicity. In: Kostrzewa R, editor. Handbook of Neurotoxicity. Springer, New York, NY. 2014.
Ross SB, Stenfors C. DSP4, a selective neurotoxin for the locus coeruleus noradrenergic system. A review of its mode of action. Neurotox Res. 2015;27:15–30.
Cassano T, Gaetani S, Morgese MG, Macheda T, Laconca L, Dipasquale P, et al. Monoaminergic changes in locus coeruleus and dorsal raphe nucleus following noradrenaline depletion. Neurochem Res. 2009;34:1417–26.
Wang RY, Gallager DW, Aghajanian GK. Stimulation of pontine reticular formation suppresses firing of serotonergic neuronses in the dorsal raphe. Nature. 1976;264:365–8.
Anderson CD, Pasquier DA, Forbes WB, Morgane PJ. Locus coeruleus-to-dorsal raphe input examined by electrophysiological and morphological methods. Brain Res Bull. 1977;2:209–21.
Aghajanian GK, Wang RY. Habenular and other midbrain raphe afferents demonstrated by a modified retrograde tracing technique. Brain Res. 1977;122:229–42.
Peyron C, Luppi PH, Fort P, Rampon C, Jouvet M. Lower brainstem catecholamine afferents to the rat dorsal raphe nucleus. J Comp Neurol. 1996;364:402–13.
Luppi P-H, Aston-Jones G, Akaoka H, Chouvet G, Jouvet M. Afferent projections to the rat locus coeruleus demonstrated by retrograde and anterograde tracing with cholera-toxin B subunit and Phaseolus vulgaris leucoagglutinin. Neuroscience. 1995;65:119–60.
Kim M-A, Lee HS, Lee BY, Waterhouse BD. Reciprocal connections between subdivisions of the dorsal raphe and the nuclear core of the locus coeruleus in the rat. Brain Res. 2004;1026:56–67.
Schwarz LA, Miyamichi K, Gao XJ, Beier KT, Weissbourd B, DeLoach KE, et al. Viral-genetic tracing of the input–output organization of a central norepinephrine circuit. Nature. 2015;524:88–92.
Schwarz LA, Luo L. Organization of the locus coeruleus-norepinephrine system. Curr Biol. 2015;25:R1051–R1056.
Altman JD, Trendelenburg AU, MacMillan L, Bernstein D, Limbird L, Starke K, et al. Abnormal regulation of the sympathetic nervous system in α2A-adrenergic receptor knockout mice. Mol Pharmacol. 1999;56:154–61.
Palij P, Stamford JA. Real-time monitoring of endogenous noradrenaline release in rat brain slices using fast cyclic voltammetry: 1. Characterisation of evoked noradrenaline efflux and uptake from nerve terminals in the bed nucleus of stria terminalis, pars ventralis. Brain Res. 1992;587:137–46.
Callado LF, Stamford JA. α2A- But not α2B/C-adrenoceptors modulate noradrenaline release in rat locus coeruleus: voltammetric data. Eur J Pharmacol. 1999;366:35–9.
Garratt JC, Crespi F, Mason R, Marsden CA. Effects of idazoxan on dorsal raphe 5-hydroxytryptamine neuronal function. Eur J Pharmacol. 1991;193:87–93.
Freedman JE, Aghajanian GK. Idazoxan (RX 781094) selectively antagonizes α2-adrenoceptors on rat central neurons. Eur J Pharmacol. 1984;105:265–72.
Haddjeri N, Blier P, de Montigny C. Effect of the alpha-2 adrenoceptor antagonist mirtazapine on the 5-hydroxytryptamine system in the rat brain. J Pharmacol Exp Ther. 1996;277:861–71.
Ingram S, Wilding TJ, McCleskey EW, Williams JT. Efficacy and kinetics of opioid action on acutely dissociated neurons. Mol Pharmacol. 1997;52:136–43.
Schwartz DD. Activation of alpha-2 adrenergic receptors inhibits norepinephrine release by a pertussis toxin-insensitive pathway independent of changes in cytosolic calcium in cultured rat sympathetic neurons. J Pharmacol Exp Ther. 1997;282:248–55.
McCune SK, Voigt MM, Hill JM. Expression of multiple alpha adrenergic receptor subtype messenger RNAs in the adult rat brain. Neuroscience. 1993;57:143–51.
Hopwood SE, Stamford JA. Noradrenergic modulation of serotonin release in rat dorsal and median raphé nuclei via alpha(1) and alpha(2A) adrenoceptors. Neuropharmacology. 2001;41:433–42.
Huang KW, Ochandarena NE, Philson AC, Hyun M, Birnbaum JE, Cicconet M, et al. Molecular and anatomical organization of the dorsal raphe nucleus. Elife. 2019;8:641.
Beenhakker MP, Huguenard JR. Astrocytes as gatekeepers of GABAB receptor function. J Neurosci. 2010;30:15262–76.
Otis TS, Mody I. Differential activation of GABAA and GABAB receptors by spontaneously released transmitter. J Neurophysiol. 1992;67:227–35.
Lee A, Rosin DL, Bockstaele EJV. alpha2A-adrenergic receptors in the rat nucleus locus coeruleus: subcellular localization in catecholaminergic dendrites, astrocytes, and presynaptic axon terminals. Brain Res. 1998;795:157–69.
Li YW, Bayliss DA. Activation of alpha 2-adrenoceptors causes inhibition of calcium channels but does not modulate inwardly-rectifying K+ channels in caudal raphe neurons. NSC. 1998;82:753–65.
Yoshioka M, Matsumoto M, Togashi H, Smith CB, Saito H. Alpha 2-adrenoceptor modulation of 5-HT biosynthesis in the rat brain. Neurosci Lett. 1992;139:53–6.
Langer SZ. α2-Adrenoceptors in the treatment of major neuropsychiatric disorders. Trends Pharmacol Sci. 2015;36:196–202.
Ordway GA, Widdowson PS, Smith KS, Halaris A. Agonist binding to α 2 ‐adrenoceptors is elevated in the locus coeruleus from victims of suicide. J Neurochem. 1994;63:617–24.
Ordway GA, Schenk J, Stockmeier CA, May W, Klimek V. Elevated agonist binding to alpha2-adrenoceptors in the locus coeruleus in major depression. Biol Psychiatry. 2003;53:315–23.
González AM, Pascual J, Meana JJ, Barturen F, Arco CD, Pazos A, et al. Autoradiographic demonstration of increased α2‐adrenoceptor agonist binding sites in the hippocampus and frontal cortex of depressed suicide victims. J Neurochem. 1994;63:256–65.
Cottingham C, Wang Q. α2 adrenergic receptor dysregulation in depressive disorders: implications for the neurobiology of depression and antidepressant therapy. Neurosci Biobehav Rev. 2012;36:2214–25.
McIntyre RS, Alsuwaidan M, Baune BT, Berk M, Demyttenaere K, Goldberg JF, et al. Treatment‐resistant depression: definition, prevalence, detection, management, and investigational interventions. World Psychiatry. 2023;22:394–412.
Gurguis GNM, Vo SP, Griffith JM, Rush AJ. Platelet alpha2A-adrenoceptor function in major depression: Gi coupling, effects of imipramine and relationship to treatment outcome. Psychiatry Res. 1999;89:73–95.
Acknowledgements
A portion was supported by the Intramural Research Program at the National Institute on Drug Abuse (H.S.H and S.C.G). The opinions expressed in this article are the authors’ own and do not reflect the views of the NIH/DHHS. The present address for H.S.H. is in the Department of Psychology, University of Washington, Seattle, Washington, 98195, USA. We would like to thank Drs. Yeka Aponte (NIH) and Brenton Laing (University of Mississippi) for providing materials and expertise for the retrograde labeling experiments. We would like to thank the Gantz lab members, Jacqueline Khamma, Nora O’Prey, Addison Eckard, Jeff Rudman, Kathryn Cochrane, and Andrew Kain for their technical assistance in c-fos immunohistochemistry and analysis.
Funding
This research was funded by a startup award from the University of Iowa Carver College of Medicine to S.C.G. and the Carver College of Medicine and Iowa Neuroscience Institute Carver Trust Early-Stage Investigator award to S.C.G.
Author information
Authors and Affiliations
Contributions
Aleigha Gugel: Substantial contributions to the acquisition, analysis, or interpretation of data for the work; Drafting the work or revising it critically for important intellectual content; Final approval of the version to be published; and Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Erik A. Ingebretsen: Substantial contributions to the acquisition, analysis, or interpretation of data for the work; Final approval of the version to be published; and Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Holly S. Hake: Substantial contributions to the acquisition, analysis, or interpretation of data for the work; Final approval of the version to be published; and Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Stephanie C. Gantz: Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; Drafting the work or revising it critically for important intellectual content; Final approval of the version to be published; and Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gugel, A., Ingebretsen, E.A., Hake, H.S. et al. LC-derived excitatory synaptic transmission to dorsal raphe serotonin neurons is inhibited by activation of alpha2-adrenergic receptors. Neuropsychopharmacol. 49, 1014–1023 (2024). https://doi.org/10.1038/s41386-024-01824-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-024-01824-3