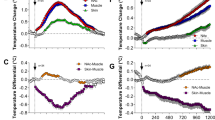
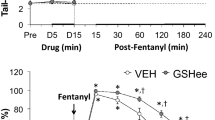
Abstract
Xylazine, a veterinary tranquillizer known by drug users as “Tranq”, is being increasingly detected in people who overdose on opioid drugs, indicating enhanced health risk of fentanyl-xylazine mixtures. We recently found that xylazine potentiates fentanyl- and heroin-induced brain hypoxia and eliminates the rebound-like post-hypoxic oxygen increases. Here, we used oxygen sensors coupled with high-speed amperometry in rats of both sexes to explore the treatment potential of naloxone plus atipamezole, a selective α2-adrenoceptor antagonist, in reversing brain (nucleus accumbens) and periphery (subcutaneous space) hypoxia induced by a fentanyl-xylazine mixture. Pretreatment with naloxone (0.2 mg/kg, IV) fully blocked brain and peripheral hypoxia induced by fentanyl (20 μg/kg, IV), but only partially decreased hypoxia induced by a fentanyl-xylazine mixture. Pretreatment with atipamezole (0.25 mg/kg, IV) fully blocked the hypoxic effects of xylazine (1.0 mg/kg, IV), but not fentanyl. Pretreatment with atipamezole + naloxone was more potent than naloxone alone in blocking the hypoxic effects of the fentanyl-xylazine mixture. Both naloxone and naloxone + atipamezole, delivered at the peak of brain hypoxia (3 min post fentanyl-xylazine exposure), reversed the rapid initial brain hypoxia, but only naloxone + atipamezole decreased the prolonged weaker hypoxia. There were no sex differences in the effects of the different drugs and their combinations on brain and peripheral oxygen responses. Results indicate that combined treatment with naloxone and atipamezole is more effective than naloxone alone in reversing the hypoxic effects of fentanyl-xylazine mixtures. Naloxone + atipamezole treatment should be considered in preventing overdoses induced by fentanyl-xylazine mixtures in humans.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 13 print issues and online access
$259.00 per year
only $19.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Raw data and the results of their primary analyses are available on request from Dr. Eugene A. Kiyatkin (NIDA-IRP, NIH; ekiyatki@intra.nida.nih.gov).
References
Butelman ER, Huang, Y, Epstein DH, Shaham Y, Goldstein RZ, Volkow ND, et al. Overdose mortality rates for opioids and stimulant drugs are substantially higher in men than in women: state-level analysis. Neuropsychopharmacology. 2023; https://doi.org/10.1038/s41386-023-01601-8.
Kariisa M, O’Donnel J, Kumar S, Mattson CL, Goldberger BA. Illicitly manufactured fentanyl-involved overdose death with detected xylazine – United States, January 2019-June 2022. CDC Report 72 (No 26).
Friedman J, Montero F, Bourgois P, Wahbi R, Dye D, et al. Xylazine spreads across the US: a growing component of the increasingly synthetic and polysubstance overdose crisis. Drug Alcohol Depend. 2022;233:109380.
Dahan A, Yassen A, Bijl H, Romberg R, Sarton E, Teppema L, et al. 2005. Comparison of the respiratory effects of intravenous buprenorphine and fentanyl in humans and rats. Br J Anaesth. 2005;94:825–34.
Yeadon M, Kitchen I. Opioids and respiration. Prog Neurobiol. 1989;33:1–16.
Gupta K, Prasad A, Nagappa M, Wong J, Abrahamyan L, Chung FF. Risk factors for opioid-induced respiratory depression and failure to rescue: a review. Curr Opin Anesthesiol. 2018;31:110–19.
MacKenzie M, Zed PJ, Ensom MHH. Opioid pharmacokinetics-pharmacodynamics: clinical implications in acute pain management in trauma. Ann Pharmacother. 2016;50:209–18.
Skolnick P. On the front lines of the opioid epidemic: rescue by naloxone. Eur J Pharmacol. 2018;835:147–53.
Skolnick P. Treatment of overdose in the synthetic opioid era. Pharmacol Ther. 2021:108019. https://doi.org/10.1016/j.pharmthera.2021.108019.
Lynn RR, Galinkin JL. Naloxone dosage for opioid reversal: current evidence and clinical implications. Ther Adv Drug Saf. 2018;9:63–88.
Moss RB, Carlo DJ. Higher doses of naloxone are needed in the synthetic opioid era. Subst Abuse Treat Prev Policy. 2019;14:6. https://doi.org/10.1186/s13011-019-0195-4.
Curay CM, Irwin MR, Kiyatkin EA. The pattern of brain oxygen response induced by intravenous fentanyl limits the time window of therapeutic efficacy of naloxone. Neuropharmacology. 2023;231:109507. https://doi.org/10.1016/j.neuropharm.2023.109507.
Compton VM, Valentino RJ, DuPont RL. Polysubstance use in the U.S. opioid crisis. Mol Psychiatry. 2021;26:41–50.
Greene SA, Thurmon JC. Xylazine—a review of its pharmacology and use in veterinary medicine. J Vet Pharmacol Ther. 1988;11:295–313.
Reyes JC, Negron JL, Colob HM, Padilla AM, Millan MY, et al. The emerging of xylazine as a new drug of abuse and its health consequences among drug users in Puerto Rico. J Urban Health. 2012;89:519–26.
Capraro AJ, Wiley JF, Tucker JR. Severe intoxication from xylazine inhalation. Pediatr Emerg Care. 2001;17:447–8.
Schwartz DD, Clark TP. Affinity of detomidine, medetomidine and xylazine for alpha-2 adrenergic receptor subtypes. J Vet Pharmacol Ther. 1998;21:107–11.
Choi S, Irwin MR, Kiyatkin EA. Xylazine effects on opioid-induced brain hypoxia. Psychopharmacology. 2023;240:1561–71. https://doi.org/10.1007/s00213-023-06390-y.
Kiyatkin EA. Physiological and drug-induced fluctuations in brain oxygen and glucose assessed by substrate-sensitive sensors coupled with high-speed amperometry. In: Wilson GS, Michael AC, editors. Compendium of in vivo monitoring in real-time molecular neuroscience. 3. Probing brain functions, disease and Injury with enhanced optical and electrochemical sensors. World Scientific; 2019. p. 219–50.
Badiani A, Belin D, Epstein D, Calu D, Shaham Y. Opiate versus psychostimulant addiction: the differences do matter. Nat Rev Neurosci. 2011;12:685–700.
Mogenson GJ, Jones DL, Yim CY. From motivation to action: functional interface between the limbic system and the motor system. Prog Neurobiol. 1980;14:69–97.
Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol Rev. 1987;94:469–92.
Solis E Jr, Cameron-Burr KT, Shaham Y, Kiyatkin EA. Fentanyl-induced brain hypoxia triggers brain hyperglycemia and biphasic changes in brain temperature. Neuropsychopharmacology. 2018;43:810–9.
Thomas SA, Curay CM, Kiyatkin EA. Relationships between oxygen changes in the brain and periphery following physiological activation and the action of heroin and cocaine. Sci Rep. 2021;11:6355.
Paxinos G, Watson C. The rat brain in stereotaxic coordinates. San Diego: Academic Press; 1998.
Bolger FB, Bennett R, Lowry JP. An in vitro characterization comparing carbon paste and Pt microelectrodes for real-time detection of brain tissue oxygen. Analyst. 2011;136:4028–35.
Wade CL, Vendruscolo LF, Schlosburg JE, Hernandez DO, Koob GF. Compulsive-like responding for opioid analgesics in rats with extended access. Neuropsychopharmacology. 2015;40:421–48.
Reiner DJ, Lofaro OM, Applebey SV, Kohan H, Venniro M, et al. Role of projections between piriform cortex and orbitofrontal cortex in relapse to fentanyl seeking after palatible food choice-induced voluntary abstinence. J Neurosci. 2020;40:2485–97.
Claypool SM, Reiner DJ, Behdin S, Orihuel J, Batista A, Caldwell K, et al. Role of piriform cortex and its afferent projections in relapse to fentanyl seeking after food choice-induced voluntary absinence. J Neurosci. 2023;43:2597–614.
Xylazine hydrochloride 1999; Committee for veterinary medical products. Xylazine Hydrochloride: Summary report. The European Agency for the Evaluation of Medicinal Products. https://www.ema.europa.eu/en/documents/mrl-report/xylazine-hydrochloride-summary-report-1-committeeveterinary-medicinal-products_en.pdf.
Perekopskiy D, Afzal A, Jackson SN, Muller L, Woods AS, Kiyatkin EA. The role of peripheral opioid receptors in triggering heroin-induced brain hypoxia. Sci Rep. 2020;10:833.
Pertovaara A, Haapalinna A, Sirvio J, Virtanen R. Pharmacological properties, central nervous system effects, and potential therapeutic applications of atipamezole, a selective alpha2-adrenoceptor antagonist. CNS Drug Rev. 2005;11:273–88.
Bola RA, Kiyatkin EA. Brain temperature effects of intravenous heroin: state dependency, environmental modulation, and the effects of dose. Neuropharmacology. 2017;126:271–80.
Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, Newman EA. Glial and neuronal control of brain blood flow. Nature. 2010;468:232–43.
Battisti-Charbonney A, Fisher J, Duffin J. The cerebrovascular response to carbon dioxide in humans. J Physiol. 2011;589:3039–48.
Schmidt CF, Kety SS. Recent studies of cerebral blood flow and cerebral metabolism in man. Trans Assoc Am Physicians. 1947;60:52–58.
Kiyatkin EA. Functional role of peripheral vasoconstriction: not only thermoregulation but much more. J Integr Neurosci. 2021;20:755–64.
Sanford TD, Colby ED. Effect of xylazine and ketamine on blood pressure, heart rate and respiratory rate in rabbits. Lab Anim Sci. 1980;30:519–23.
Kanawati S, Yaksh TL, Anderson RE, Marsh RW. Effects of clonidine on cerebral blood flow and the response to arterial CO2. J Cereb Blood Flow Metab. 1986;6:358–65.
Drew GM. Effects of alpha-adrenergic agonists and antagonists on pre- and postsynaptically located alpha-adrenoceptors. Eur J Pharmacol. 1976;36:313–20.
Kobinger W. Central alpha-adrenergic systems as target for hypotensive drugs. Rev Physiol Biochem Pharmacol. 1978;81:39–100.
Blasig J, Herz A, Reinhold K, Zieglgansberger S. Development of physical dependence on morphine in respect to time and dosage and quantification of the precipitated withdrawal syndrome in rats. Psychopharmacologia. 1973;33:19–38.
Bossert JM, Kiyatkin EA, Korah H, Hoots JK, Afzal A, et al. In a rat model of opioid maintenance, the G Protein-biased mu opioid receptor agonists TRV130 decreases relapse to oxycodone seeking and taking and prevents oxycodone-induced brain hypoxia. Biol Psychiatry. 2020;88:935–44.
Schmidt-Nielson K. Animal physiology: adaptation and environment. Cambridge: Cambridge University Press; 1989.
Trescot AM, Datta S, Lee M, Hansen H. Opioid pharmacology. Pain Physician. 2008;11:S133–53.
Ohtsuka H, Fujita K, Kobayashi H. Pharmacokinetics of fentanyl in male and female rats after intravenous administration. ArzneimForschDrugRes. 2007;57:260–63.
Acknowledgements
The study was supported by the Intramural Research Program of the NIH, NIDA.
Funding
The study was supported by the Intramural Research Program of the NIH, NIDA (1ZIADA000566-12 [EAK]; 1ZIADA000434-17 [YS]).
Author information
Authors and Affiliations
Contributions
EAK: Conceptualization, Surgery procedures, Participation in experiments, Data analyses, Writing the manuscript; SC, MRI and MN: Conceptualization, Performance of experiments, Data analyses, Graphic work, Histological work, Review and editing the manuscript. YS: Conceptualization, Writing, and editing the manuscript.
Corresponding author
Ethics declarations
Competing interests
YS is an Associate (Reviewing) Editor for Neuropsychopharmacology. The other authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Choi, S., Irwin, M.R., Noya, M.R. et al. Combined treatment with naloxone and the alpha2 adrenoceptor antagonist atipamezole reversed brain hypoxia induced by a fentanyl-xylazine mixture in a rat model. Neuropsychopharmacol. (2023). https://doi.org/10.1038/s41386-023-01782-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41386-023-01782-2