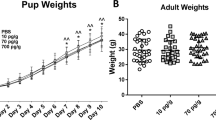
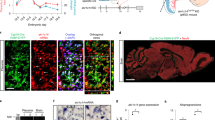
Abstract
Psychiatric and obstetric diseases are growing threats to public health and share high rates of co-morbidity. G protein-coupled receptor signaling (e.g., vasopressin, serotonin) may be a convergent psycho-obstetric risk mechanism. Regulator of G Protein Signaling 2 (RGS2) mutations increase risk for both the gestational disease preeclampsia and for depression. We previously found preeclampsia-like, anti-angiogenic obstetric phenotypes with reduced placental Rgs2 expression in mice. Here, we extend this to test whether conserved cerebrovascular and serotonergic mechanisms are also associated with risk for neurobiological phenotypes in the Rgs2 KO mouse. Rgs2 KO exhibited anxiety-, depression-, and hedonic-like behaviors. Cortical vascular density and vessel length decreased in Rgs2 KO; cortical and white matter thickness and cell densities were unchanged. In Rgs2 KO, serotonergic gene expression was sex-specifically changed (e.g., cortical Htr2a, Maoa increased in females but all serotonin targets unchanged or decreased in males); redox-related expression increased in paraventricular nucleus and aorta; and angiogenic gene expression was changed in male but not female cortex. Whole-cell recordings from dorsal raphe serotonin neurons revealed altered 5-HT1A receptor-dependent inhibitory postsynaptic currents (5-HT1A-IPSCs) in female but not male KO neurons. Additionally, serotonin transporter blockade by the SSRI sertraline increased the amplitude and time-to-peak of 5-HT1A-IPSCs in KO neurons to a greater extent than in WT neurons in females only. These results demonstrate behavioral, cerebrovascular, and sertraline hypersensitivity phenotypes in Rgs2 KOs, some of which are sex-specific. Disruptions may be driven by vascular and cell stress mechanisms linking the shared pathogenesis of psychiatric and obstetric disease to reveal future targets.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 13 print issues and online access
$259.00 per year
only $19.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
O’Connor E, Rossom RC, Henninger M, Groom HC, Burda BU, Henderson JT, et al. Screening for Depression in Adults: An Updated Systematic Evidence Review for the U.S. Preventive Services Task Force. 2016.
Trost SL, Beauregard JL, Smoots AN, Ko JY, Haight SC, Moore Simas TA, et al. Preventing Pregnancy-Related Mental Health Deaths: Insights From 14 US Maternal Mortality Review Committees, 2008-17. Health Aff (Millwood). 2021;40:1551–59.
Shuman CJ, Peahl AF, Pareddy N, Morgan ME, Chiangong J, Veliz PT, et al. Postpartum depression and associated risk factors during the COVID-19 pandemic. BMC Res Notes. 2022;15:102.
Bonari L, Bennett H, Einarson A, Koren G. Risks of untreated depression during pregnancy. Can Fam Physician. 2004;50:37–9.
Goldman-Mellor S, Margerison CE. Maternal drug-related death and suicide are leading causes of postpartum death in California. Am J Obstet Gynecol. 2019;221:489e1–89e9.
Liu X, Jin G, Fan B, Xing Y, Wang L, Wang M, et al. The impact of ALDH2 activation by Alda-1 on the expression of VEGF in the hippocampus of a rat model of post-MI depression. Neurosci Lett. 2018;674:156–61.
Marcus SM. Depression during pregnancy: rates, risks and consequences–Motherisk Update 2008. Can J Clin Pharmacol. 2009;16:e15–22.
Bunevicius R, Kusminskas L, Bunevicius A, Nadisauskiene RJ, Jureniene K, Pop VJ. Psychosocial risk factors for depression during pregnancy. Acta Obstet Gynecol Scand. 2009;88:599–605.
Silverman ME, Reichenberg A, Savitz DA, Cnattingius S, Lichtenstein P, Hultman CM, et al. The risk factors for postpartum depression: A population-based study. Depress Anxiety. 2017;34:178–87.
Vignato JA, Gumusoglu SB, Davis HA, Scroggins SM, Hamilton WS, Brandt DS, et al. Selective Serotonin Reuptake Inhibitor Use in Pregnancy and Protective Mechanisms in Preeclampsia. Reprod Sci. 2022; 30:701–12
Qiu C, Sanchez SE, Lam N, Garcia P, Williams MA. Associations of depression and depressive symptoms with preeclampsia: results from a Peruvian case-control study. BMC Womens Health. 2007;7:15.
Caropreso L, de Azevedo Cardoso T, Eltayebani M, Frey BN. Preeclampsia as a risk factor for postpartum depression and psychosis: a systematic review and meta-analysis. Arch Womens Ment Health. 2020;23:493–505.
Palmsten K, Setoguchi S, Margulis AV, Patrick AR, Hernández-Díaz S. Elevated risk of preeclampsia in pregnant women with depression: depression or antidepressants? Am J Epidemiol. 2012;175:988–97.
Srajer A, Johnson JA, Yusuf K. Preeclampsia and postpartum mental health: mechanisms and clinical implications. J Matern Fetal Neonatal Med. 2022;35:8443–49.
Bolte AC, van Geijn HP, Dekker GA. Pathophysiology of preeclampsia and the role of serotonin. Eur J Obstet Gynecol Reprod Biol. 2001;95:12–21.
Lang U, Prada J, Clark KE. Systemic and uterine vascular response to serotonin in third trimester pregnant ewes. Eur J Obstet Gynecol Reprod Biol. 1993;51:131–8.
Bodelsson G, Marsál K, Stjernquist M. Reduced contractile effect of endothelin-1 and noradrenalin in human umbilical artery from pregnancies with abnormal umbilical artery flow velocity waveforms. Early Hum Dev. 1995;42:15–28.
Feng X, Zhang Y, Tao J, Lu L, Liu J, Zhao M, et al. Comparisons of vascular responses to vasoconstrictors in human placenta in preeclampsia between preterm and later term. Curr Pharm Biotechnol. 2019; 21:727–33
Gumusoglu S, Scroggins S, Vignato J, Santillan D, Santillan M. The Serotonin-Immune Axis in Preeclampsia. Curr Hypertens Rep. 2021;23:37.
Santillan MK, Santillan DA, Scroggins SM, Min JY, Sandgren JA, Pearson NA, et al. Vasopressin in preeclampsia: a novel very early human pregnancy biomarker and clinically relevant mouse model. Hypertension. 2014;64:852–9.
Sandgren JA, Scroggins SM, Santillan DA, Devor EJ, Gibson-Corley KN, Pierce GL, et al. Vasopressin: the missing link for preeclampsia? Am J Physiol Regul Integr Comp Physiol. 2015;309:R1062–4.
Kashkouli M, Jahanian Sadatmahalleh S, Ziaei S, Kazemnejad A, Saber A, Darvishnia H, et al. Relationship between postpartum depression and plasma vasopressin level at 6-8 weeks postpartum: a cross-sectional study. Sci Rep. 2023;13:3518.
Gumusoglu S, Davis L, Schickling B, Devor E, Von Tersch L, Santillan M, et al. Effects of maternal hypertension on cord blood Arginine vasopressin receptor expression. Pregnancy Hypertens. 2023;31:1–3.
McNestry C, Killeen SL, Crowley RK, McAuliffe FM. Pregnancy complications and later life women’s health. Acta Obstet Gynecol Scand. 2023;102:523–31.
Szczepanska-Sadowska E. The Heart as a Target of Vasopressin and Other Cardiovascular Peptides in Health and Cardiovascular Diseases. Int J Mol Sci. 2022;23:14414.
Golyszny M, Obuchowicz E. Are neuropeptides relevant for the mechanism of action of SSRIs? Neuropeptides. 2019;75:1–17.
Okimoto N, Bosch OJ, Slattery DA, Pflaum K, Matsushita H, Wei FY, et al. RGS2 mediates the anxiolytic effect of oxytocin. Brain Res. 2012;1453:26–33.
Smoller JW, Paulus MP, Fagerness JA, Purcell S, Yamaki LH, Hirshfeld-Becker D, et al. Influence of RGS2 on anxiety-related temperament, personality, and brain function. Arch Gen Psych. 2008;65:298–308.
Perschbacher KJ, Deng G, Fisher RA, Gibson-Corley KN, Santillan MK, Grobe JL. Regulators of G protein signaling in cardiovascular function during pregnancy. Physiol Genomics. 2018;50:590–604.
Karppanen T, Kaartokallio T, Klemetti MM, Heinonen S, Kajantie E, Kere J, et al. An RGS2 3’UTR polymorphism is associated with preeclampsia in overweight women. BMC Genet. 2016;17:121.
Kvehaugen AS, Melien O, Holmen OL, Laivuori H, Dechend R, Staff AC. Hypertension after preeclampsia and relation to the C1114G polymorphism (rs4606) in RGS2: data from the Norwegian HUNT2 study. BMC Med Genet. 2014;15:28.
Kvehaugen AS, Melien O, Holmen OL, Laivuori H, Oian P, Andersgaard AB, et al. Single nucleotide polymorphisms in G protein signaling pathway genes in preeclampsia. Hypertension. 2013;61:655–61.
Perschbacher KJ, Deng G, Sandgren JA, Walsh JW, Witcher PC, Sapouckey SA, et al. Reduced mRNA Expression of RGS2 (Regulator of G Protein Signaling-2) in the Placenta Is Associated With Human Preeclampsia and Sufficient to Cause Features of the Disorder in Mice. Hypertension. 2020;75:569–79.
Koch JN, Dahlen SA, Owens EA, Osei-Owusu P. Regulator of G Protein Signaling 2 Facilitates Uterine Artery Adaptation During Pregnancy in Mice. J Am Heart Assoc. 2019;8:e010917.
Jie L, Owens EA, Plante LA, Fang Z, Rensing DT, Moeller KD, et al. RGS2 squelches vascular Gi/o and Gq signaling to modulate myogenic tone and promote uterine blood flow. Physiol Rep. 2016;4:e12692.
Stein MB, Keshaviah A, Haddad SA, Van Ameringen M, Simon NM, Pollack MH, et al. Influence of RGS2 on sertraline treatment for social anxiety disorder. Neuropsychopharmacology. 2014;39:1340–6.
Leygraf A, Hohoff C, Freitag C, Willis-Owen SA, Krakowitzky P, Fritze J, et al. Rgs 2 gene polymorphisms as modulators of anxiety in humans? J Neural Transm (Vienna). 2006;113:1921–5.
Hohoff C, Weber H, Richter J, Domschke K, Zwanzger PM, Ohrmann P, et al. RGS2 ggenetic variation: association analysis with panic disorder and dimensional as well as intermediate phenotypes of anxiety. Am J Med Genet B Neuropsychiatr Genet. 2015;168B:211–22.
Mouri K, Hishimoto A, Fukutake M, Nishiguchi N, Shirakawa O, Maeda K. Association study of RGS2 gene polymorphisms with panic disorder in Japanese. Kobe J Med Sci. 2010;55:E116–21.
Amstadter AB, Koenen KC, Ruggiero KJ, Acierno R, Galea S, Kilpatrick DG, et al. Variant in RGS2 moderates posttraumatic stress symptoms following potentially traumatic event exposure. J Anxiety Disord. 2009;23:369–73.
Williams AV, Peña CJ, Ramos-Maciel S, Laman-Maharg A, Ordoñez-Sanchez E, Britton M, et al. Comparative Transcriptional Analyses in the Nucleus Accumbens Identifies RGS2 as a Key Mediator of Depression-Related Behavior. Biol Psych. 2022;92:942–51.
Amstadter AB, Koenen KC, Ruggiero KJ, Acierno R, Galea S, Kilpatrick DG, et al. Variation in RGS2 is associated with suicidal ideation in an epidemiological study of adults exposed to the 2004 Florida hurricanes. Arch Suicide Res. 2009;13:349–57.
Lifschytz T, Broner EC, Zozulinsky P, Slonimsky A, Eitan R, Greenbaum L, et al. Relationship between Rgs2 gene expression level and anxiety and depression-like behaviour in a mutant mouse model: serotonergic involvement. Int J Neuropsychopharmacol. 2012;15:1307–18.
Raab A, Popp S, Lesch KP, Lohse MJ, Fischer M, Deckert J, et al. Increased fear learning, spatial learning as well as neophobia in Rgs2. Genes Brain Behav. 2018;17:e12420.
Oliveira-Dos-Santos AJ, Matsumoto G, Snow BE, Bai D, Houston FP, Whishaw IQ, et al. Regulation of T cell activation, anxiety, and male aggression by RGS2. Proc Natl Acad Sci. 2000;97:12272–7.
Luo Y, Kataoka Y, Ostinelli EG, Cipriani A, Furukawa TA. National Prescription Patterns of Antidepressants in the Treatment of Adults With Major Depression in the US Between 1996 and 2015: A Population Representative Survey Based Analysis. Front Psych. 2020;11:35.
Desaunay P, Eude LG, Dreyfus M, Alexandre C, Fedrizzi S, Alexandre J, et al. Benefits and Risks of Antidepressant Drugs During Pregnancy: A Systematic Review of Meta-analyses. Paediatr Drugs. 2023;25:247–65.
Gumusoglu SB, Schickling BM, Vignato JA, Santillan DA, Santillan MK. Selective serotonin reuptake inhibitors and preeclampsia: A quality assessment and meta-analysis. Pregnancy Hypertens. 2022;30:36–43.
Tatsumi M, Groshan K, Blakely RD, Richelson E. Pharmacological profile of antidepressants and related compounds at human monoamine transporters. Eur J Pharmacol. 1997;340:249–58.
Gumusoglu SB, Chilukuri ASS, Santillan DA, Santillan MK, Stevens HE. Neurodevelopmental Outcomes of Prenatal Preeclampsia Exposure. Trends Neurosci. 2020;43:253–68.
Gumusoglu SB, Fine RS, Murray SJ, Bittle JL, Stevens HE. The role of IL-6 in neurodevelopment after prenatal stress. Brain Behav Immun. 2017;65:274–83.
Gumusoglu SB, Hing BWQ, Chilukuri ASS, Dewitt JJ, Scroggins SM, Stevens HE. Chronic maternal interleukin-17 and autism-related cortical gene expression, neurobiology, and behavior. Neuropsychopharmacology. 2020;45:1008–17.
Gumusoglu SB, Chilukuri ASS, Hing BWQ, Scroggins SM, Kundu S, Sandgren JA, et al. Altered offspring neurodevelopment in an arginine vasopressin preeclampsia model. Transl Psych. 2021;11:79.
Fairless AH, Dow HC, Toledo MM, Malkus KA, Edelmann M, Li H, et al. Low sociability is associated with reduced size of the corpus callosum in the BALB/cJ inbred mouse strain. Brain Res. 2008;1230:211–7.
Fairless AH, Katz JM, Vijayvargiya N, Dow HC, Kreibich AS, Berrettini WH, et al. Development of home cage social behaviors in BALB/cJ vs. C57BL/6J mice. Behav Brain Res. 2013;237:338–47.
Ferri SL, Dow HC, Schoch H, Lee JY, Brodkin ES, Abel T. Age- and sex-specific fear conditioning deficits in mice lacking Pcdh10, an Autism Associated Gene. Neurobiol Learn Mem. 2021;178:107364.
Ferri SL, Kreibich AS, Torre M, Piccoli CT, Dow H, Pallathra AA, et al. Activation of basolateral amygdala in juvenile C57BL/6J mice during social approach behavior. Neuroscience. 2016;335:184–94.
Klomp AJ, Plumb A, Mehr JB, Madencioglu DA, Wen H, Williams AJ. Neuronal deletion of Ca(V)1.2 is associated with sex-specific behavioral phenotypes in mice. Sci Rep. 2022;12:22152.
Coryell MW, Wunsch AM, Haenfler JM, Allen JE, Schnizler M, Ziemann AE, et al. Acid-sensing ion channel-1a in the amygdala, a novel therapeutic target in depression-related behavior. J Neurosci. 2009;29:5381–8.
Dailey ME, Waite M. Confocal imaging of microglial cell dynamics in hippocampal slice cultures. Methods. 1999;18:222–30.
Grossmann R, Stence N, Carr J, Fuller L, Waite M, Dailey ME. Juxtavascular microglia migrate along brain microvessels following activation during early postnatal development. Glia. 2002;37:229–40.
Luna RL, Kay VR, Ratsep MT, Khalaj K, Bidarimath M, Peterson N, et al. Placental growth factor deficiency is associated with impaired cerebral vascular development in mice. Mol Hum Reprod. 2016;22:130–42.
Sandgren JA, Deng G, Linggonegoro DW, Scroggins SM, Perschbacher KJ, Nair AR, et al. Arginine vasopressin infusion is sufficient to model clinical features of preeclampsia in mice. JCI Insight. 2018;3:e99403.
Kucuk M, Kaya M, Kalayci R, Cimen V, Kudat H, Arican N, et al. Effects of losartan on the blood-brain barrier permeability in long-term nitric oxide blockade-induced hypertensive rats. Life Sci. 2002;71:937–46.
Khamma JK, Copeland DS, Hake HS, Gantz SC. Spatiotemporal Control of Noradrenaline-Dependent Synaptic Transmission in Mouse Dorsal Raphe Serotonin Neurons. J Neurosci. 2022;42:968–79.
Gantz SC, Levitt ES, Llamosas N, Neve KA, Williams JT. Depression of Serotonin Synaptic Transmission by the Dopamine Precursor L-DOPA. Cell Rep. 2015;12:944–54.
Santillan MK, Pelham CJ, Ketsawatsomkron P, Santillan DA, Davis DR, Devor EJ, et al. Pregnant mice lacking indoleamine 2,3-dioxygenase exhibit preeclampsia phenotypes. Physiol Rep. 2015;3:e12257.
Jabarin R, Netser S, Wagner S. Beyond the three-chamber test: toward a multimodal and objective assessment of social behavior in rodents. Mol Autism. 2022;13:41.
Sideromenos S, Lindtner C, Zambon A, Horvath O, Berger A, Pollak DD. VEGF Treatment Ameliorates Depression-Like Behavior in Adult Offspring After Maternal Immune Activation. Cells. 2020;9:1048.
Ghavami A, Hunt RA, Olsen MA, Zhang J, Smith DL, Kalgaonkar S, et al. Differential effects of regulator of G protein signaling (RGS) proteins on serotonin 5-HT1A, 5-HT2A, and dopamine D2 receptor-mediated signaling and adenylyl cyclase activity. Cell Signal. 2004;16:711–21.
Mark MD, Wollenweber P, Gesk A, Kösters K, Batzke K, Janoschka C, et al. RGS2 drives male aggression in mice via the serotonergic system. Commun Biol. 2019;2:373.
Christmas DM, Potokar J, Davies SJ. A biological pathway linking inflammation and depression: activation of indoleamine 2,3-dioxygenase. Neuropsychiatr Dis Treat. 2011;7:431–9.
Maes M, Leonard BE, Myint AM, Kubera M, Verkerk R. The new ‘5-HT’ hypothesis of depression: cell-mediated immune activation induces indoleamine 2,3-dioxygenase, which leads to lower plasma tryptophan and an increased synthesis of detrimental tryptophan catabolites (TRYCATs), both of which contribute to the onset of depression. Prog Neuropsychopharmacol Biol Psych. 2011;35:702–21.
Muller N, Schwarz MJ. The immune-mediated alteration of serotonin and glutamate: towards an integrated view of depression. Mol Psych. 2007;12:988–1000.
O’Connor JC, Lawson MA, Andre C, Moreau M, Lestage J, Castanon N, et al. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol Psych. 2009;14:511–22.
Williams JT, Colmers WF, Pan ZZ. Voltage- and ligand-activated inwardly rectifying currents in dorsal raphe neurons in vitro. J Neurosci. 1988;8:3499–506.
Courtney NA, Ford CP. Mechanisms of 5-HT1A receptor-mediated transmission in dorsal raphe serotonin neurons. J Physiol. 2016;594:953–65.
Redman CWG, Staff AC, Roberts JM. Syncytiotrophoblast stress in preeclampsia: the convergence point for multiple pathways. Am J Obstet Gynecol. 2022;226:S907–S27.
Harmon AC, Cornelius DC, Amaral LM, Faulkner JL, Cunningham MW Jr, Wallace K, et al. The role of inflammation in the pathology of preeclampsia. Clin Sci (Lond). 2016;130:409–19.
Cornelius DC. Preeclampsia: From Inflammation to Immunoregulation. Clin Med Insights Blood Disord. 2018;11:1179545X17752325.
D’Souza MS, Seeley SL, Emerson N, Rose-Malkamaki MJ, Ho SP, Tsai YC, et al. Attenuation of nicotine-induced rewarding and antidepressant-like effects in male and female mice lacking regulator of G-protein signaling 2. Pharmacol Biochem Behav. 2022;213:173338.
Klonoff-Cohen HS, Cross JL, Pieper CF. Job stress and preeclampsia. Epidemiology. 1996;7:245–9.
Roberts AL, Lyall K, Rich-Edwards JW, Ascherio A, Weisskopf MG. Association of maternal exposure to childhood abuse with elevated risk for autism in offspring. JAMA Psych. 2013;70:508–15.
Wang A, Rana S, Karumanchi SA. Preeclampsia: the role of angiogenic factors in its pathogenesis. Physiology (Bethesda). 2009;24:147–58.
Villa PM, Marttinen P, Gillberg J, Lokki AI, Majander K, Orden MR, et al. Cluster analysis to estimate the risk of preeclampsia in the high-risk Prediction and Prevention of Preeclampsia and Intrauterine Growth Restriction (PREDO) study. PLoS One. 2017;12:e0174399.
Koenen KC, Amstadter AB, Ruggiero KJ, Acierno R, Galea S, Kilpatrick DG, et al. RGS2 and generalized anxiety disorder in an epidemiologic sample of hurricane-exposed adults. Depress Anxiety. 2009;26:309–15.
Heximer SP. RGS2-mediated regulation of Gqalpha. Methods Enzymol. 2004;390:65–82.
Osei-Owusu P, Sabharwal R, Kaltenbronn KM, Rhee MH, Chapleau MW, Dietrich HH, et al. Regulator of G protein signaling 2 deficiency causes endothelial dysfunction and impaired endothelium-derived hyperpolarizing factor-mediated relaxation by dysregulating Gi/o signaling. J Biol Chem. 2012;287:12541–9.
Tokudome T, Otani K, Mao Y, Jensen LJ, Arai Y, Miyazaki T, et al. Endothelial Natriuretic Peptide Receptor 1 Play Crucial Role for Acute and Chronic Blood Pressure Regulation by Atrial Natriuretic Peptide. Hypertension. 2022;79:1409–22.
D’Souza MS, Luu AN, Guisinger TC, Seeley SL, Waldschmidt RA, Chrissobolis S. Regulator of G-Protein Signaling 5 Protein Deficiency Differentially Influences Blood Pressure, Vascular and Behavioral Effects in Aged Male Mice. J Cardiovasc Pharmacol. 2022;80:305–13.
Yalcin B, Willis-Owen SA, Fullerton J, Meesaq A, Deacon RM, Rawlins JN, et al. Genetic dissection of a behavioral quantitative trait locus shows that Rgs2 modulates anxiety in mice. Nat Genet. 2004;36:1197–202.
Pham H, Sieg J, Seeley SL, D’Souza MS. Differential methamphetamine-induced behavioral effects in male and female mice lacking regulator of G Protein signaling 4. Behav Brain Res. 2022;423:113770.
Rorabaugh BR, Rose MJ, Stoops TS, Stevens AA, Seeley SL, D’Souza MS. Regulators of G-protein signaling 2 and 4 differentially regulate cocaine-induced rewarding effects. Physiol Behav. 2018;195:9–19.
Rempe RG, Hartz AMS, Bauer B. Matrix metalloproteinases in the brain and blood-brain barrier: Versatile breakers and makers. J Cereb Blood Flow Metab. 2016;36:1481–507.
Tsang CK, Liu Y, Thomas J, Zhang Y, Zheng XF. Superoxide dismutase 1 acts as a nuclear transcription factor to regulate oxidative stress resistance. Nat Commun. 2014;5:3446.
Eleutherio ECA, Silva Magalhaes RS, de Araujo Brasil A, Monteiro Neto JR, de Holanda Paranhos L. SOD1, more than just an antioxidant. Arch Biochem Biophys. 2021;697:108701.
Milani P, Amadio M, Laforenza U, Dell’Orco M, Diamanti L, Sardone V, et al. Posttranscriptional regulation of SOD1 gene expression under oxidative stress: Potential role of ELAV proteins in sporadic ALS. Neurobiol Dis. 2013;60:51–60.
Milani P, Gagliardi S, Cova E, Cereda C. SOD1 Transcriptional and Posttranscriptional Regulation and Its Potential Implications in ALS. Neurol Res Int. 2011;2011:458427.
Deng Y, Dickey JE, Saito K, Deng G, Singh U, Jiang J, et al. Elucidating the role of Rgs2 expression in the PVN for metabolic homeostasis in mice. Mol Metab. 2022;66:101622.
Lan N, Hellemans KG, Ellis L, Weinberg J. Exposure to Chronic Mild Stress Differentially Alters Corticotropin-Releasing Hormone and Arginine Vasopressin mRNA Expression in the Stress-Responsive Neurocircuitry of Male and Female Rats Prenatally Exposed to Alcohol. Alcohol Clin Exp Res. 2015;39:2414–21.
Taylor PV, Veenema AH, Paul MJ, Bredewold R, Isaacs S, de Vries GJ. Sexually dimorphic effects of a prenatal immune challenge on social play and vasopressin expression in juvenile rats. Biol Sex Differ. 2012;3:15.
Goel N, Philippe TJ, Chang J, Koblanski ME, Viau V. Cellular and serotonergic correlates of habituated neuroendocrine responses in male and female rats. Psychoneuroendocrinology. 2022;136:105599.
Rood BD, Stott RT, You S, Smith CJ, Woodbury ME, De Vries GJ. Site of origin of and sex differences in the vasopressin innervation of the mouse (Mus musculus) brain. J Comp Neurol. 2013;521:2321–58.
de Souza Villa P, Menani JV, de Arruda Camargo GM, de Arruda Camargo LA, Saad WA. Activation of the serotonergic 5-HT1A receptor in the paraventricular nucleus of the hypothalamus inhibits water intake and increases urinary excretion in water-deprived rats. Regul Pept. 2008;150:14–20.
Issotina Zibrila A, Li Y, Wang Z, Zhao G, Liu H, Leng J, et al. Acetylcholinesterase inhibition with Pyridostigmine attenuates hypertension and neuroinflammation in the paraventricular nucleus in rat model for Preeclampsia. Int Immunopharmacol. 2021;101:108365.
Matsuura T, Shinohara K, Iyonaga T, Hirooka Y, Tsutsui H. Prior exposure to placental ischemia causes increased salt sensitivity of blood pressure via vasopressin production and secretion in postpartum rats. J Hypertens. 2019;37:1657–67.
Kato TM, Fujimori-Tonou N, Mizukami H, Ozawa K, Fujisawa S, Kato T. Presynaptic dysregulation of the paraventricular thalamic nucleus causes depression-like behavior. Sci Rep. 2019;9:16506.
Stanton LM, Price AJ, Manning EE. Hypothalamic corticotrophin releasing hormone neurons in stress-induced psychopathology: Revaluation of synaptic contributions. J Neuroendocrinol. 2023;35:e13268.
Oliver DK, Intson K, Sargin D, Power SK, McNabb J, Ramsey AJ, et al. Chronic social isolation exerts opposing sex-specific consequences on serotonin neuronal excitability and behaviour. Neuropharmacology. 2020;168:108015.
McEuen JG, Semsar KA, Lim MA, Bale TL. Influence of sex and corticotropin-releasing factor pathways as determinants in serotonin sensitivity. Endocrinology. 2009;150:3709–16.
Murray EJ, Gumusoglu SB, Santillan DA, Santillan MK. Manipulating CD4+ T Cell Pathways to Prevent Preeclampsia. Front Bioeng Biotechnol. 2022;9:811417.
Taylor WD, Aizenstein HJ, Alexopoulos GS. The vascular depression hypothesis: mechanisms linking vascular disease with depression. Mol Psych. 2013;18:963–74.
Funding
This work was supported by the NIH (5T32HL007121-45 to SBG; HD089940, HD000849, RR024980, 3UL1TR002537, P50HD10355601A1 to MKS; T32 HL007344 to BMS), the American Heart Association (AHA) (18SCG34350001 and 19IPLOI34760288 to MKS; 22POST30908921 to SBG), a startup award from the University of Iowa Carver College of Medicine (SCG), and the Carver College of Medicine and Iowa Neuroscience Institute Carver Trust Early-Stage Investigator award (to SCG).
Author information
Authors and Affiliations
Contributions
SBG: Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; Drafting the work or revising it critically for important intellectual content; final approval of the version to be published; and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. MKS: Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work. AG: Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work. BS: Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; Drafting the work or revising it critically for important intellectual content; final approval of the version to be published; KW: Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work. ML: Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; Drafting the work or revising it critically for important intellectual content. Hannah Sullivan: Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work. KC: Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work. BB: Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work. MKS: Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work. YZ: Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work. ED: Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; and final approval of the version to be published. DAS: Drafting the work or revising it critically for important intellectual content; final approval of the version to be published; and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. SCG: Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; Drafting the work or revising it critically for important intellectual content; final approval of the version to be published; and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. MKS: Drafting the work or revising it critically for important intellectual content; final approval of the version to be published; and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Competing interests
MKS is a member of the Medical Advisory Board for Comanche Pharmaceuticals and EndPreeclampsia, LLC. DAS is a member of the Medical Advisory Board for EndPreeclampsia, LLC. DAS and MKS hold patents related to the prediction and treatment of preeclampsia: US 293 #9,937,182 (April 10, 2018), EU #2,954,324, and PCT/US2018/027152. All other authors report no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gumusoglu, S.B., Kiel, M.D., Gugel, A. et al. Anti-angiogenic mechanisms and serotonergic dysfunction in the Rgs2 knockout model for the study of psycho-obstetric risk. Neuropsychopharmacol. 49, 864–875 (2024). https://doi.org/10.1038/s41386-023-01749-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-023-01749-3