Abstract
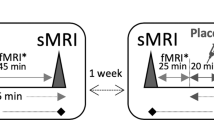
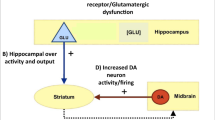
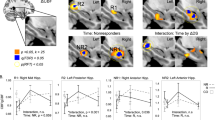
Hippocampal hyperactivity is a novel pharmacological target in the treatment of schizophrenia. We hypothesized that levetiracetam (LEV), a drug binding to the synaptic vesicle glycoprotein 2 A, normalizes hippocampal activity in persons with schizophrenia and can be measured using neuroimaging methods. Thirty healthy control participants and 30 patients with schizophrenia (28 treated with antipsychotic drugs), were randomly assigned to a double-blind, cross-over trial to receive a single administration of 500 mg oral LEV or placebo during two study visits. At each visit, we assessed hippocampal function using resting state fractional amplitude of low frequency fluctuations (fALFF), cerebral blood flow (CBF) with arterial spin labeling, and hippocampal blood-oxygen-level-dependent (BOLD) signal during a scene processing task. After placebo treatment, we found significant elevations in hippocampal fALFF in patients with schizophrenia, consistent with hippocampal hyperactivity. Additionally, hippocampal fALFF in patients with schizophrenia after LEV treatment did not significantly differ from healthy control participants receiving placebo, suggesting that LEV may normalize hippocampal hyperactivity. In contrast to our fALFF findings, we did not detect significant group differences or an effect of LEV treatment on hippocampal CBF. In the context of no significant group difference in BOLD signal, we found that hippocampal recruitment during scene processing is enhanced by LEV more significantly in schizophrenia. We conclude that pharmacological modulation of hippocampal hyperactivity in schizophrenia can be studied with some neuroimaging methods, but not others. Additional studies in different cohorts, employing alternate neuroimaging methods and study designs, are needed to establish levetiracetam as a treatment for schizophrenia.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 13 print issues and online access
$259.00 per year
only $19.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Schobel SA, Chaudhury NH, Khan UA, Paniagua B, Styner MA, Asllani I. et al. Imaging Patients with Psychosis and a Mouse Model Establishes a Spreading Pattern of Hippocampal Dysfunction and Implicates Glutamate as a Driver. Neuron. 2013;78:81–93. https://doi.org/10.1016/j.neuron.2013.02.011.
Modinos G, Şimşek F, Azis M, Bossong M, Bonoldi I, Samson C. et al. Prefrontal GABA levels, hippocampal resting perfusion and the risk of psychosis. Neuropsychopharmacology. 2018;43:2652–9. https://doi.org/10.1038/s41386-017-0004-6.
Schobel SA, Lewandowski NM, Corcoran CM, Moore H, Brown T, Malaspina D. et al. Differential targeting of the CA1 subfield of the hippocampal formation by schizophrenia and related psychotic disorders. Arch Gen Psychiatry. 2009;66:938–46. https://doi.org/10.1001/archgenpsychiatry.2009.115.
Talati P, Rane S, Kose S, Blackford JU, Gore J, Donahue MJ. et al. Increased hippocampal CA1 cerebral blood volume in schizophrenia. Neuroimage Clin. 2014;5:359–64. https://doi.org/10.1016/j.nicl.2014.07.004.
Stan AD, Ghose S, Zhao C, Hulsey K, Mihalakos P, Yanagi M. et al. Magnetic resonance spectroscopy and tissue protein concentrations together suggest lower glutamate signaling in dentate gyrus in schizophrenia. Mol Psychiatry. 2015;20:433–9. https://doi.org/10.1038/mp.2014.54.
Benes FM. Evidence for altered trisynaptic circuitry in schizophrenic hippocampus. Biol Psychiatry. 1999;46:589–99. https://doi.org/10.1016/S0006-3223(99)00136-5.
Heckers S, Konradi C. GABAergic mechanisms of hippocampal hyperactivity in schizophrenia. Schizophr Res. 2015;167:4–11. https://doi.org/10.1016/j.schres.2014.09.041.
Lodge DJ, Behrens MM, Grace AA. A loss of parvalbumin-containing interneurons is associated with diminished oscillatory activity in an animal model of schizophrenia. J Neurosci. 2009;29:2344–54. https://doi.org/10.1523/JNEUROSCI.5419-08.2009.
Grace AA, Gomes FV. The Circuitry of Dopamine System Regulation and its Disruption in Schizophrenia: Insights Into Treatment and Prevention. Schizophr Bull. 2018. https://doi.org/10.1093/schbul/sbx199.
Kiemes A, Serrano Navacerrada ME, Kim E, Randall K, Simmons C, Rojo Gonzalez L. et al. Erbb4 Deletion From Inhibitory Interneurons Causes Psychosis-Relevant Neuroimaging Phenotypes. Schizophr Bull. 2023;49:569–80. https://doi.org/10.1093/SCHBUL/SBAC192.
Lisman JE, Coyle JT, Green RW, Javitt DC, Benes FM, Heckers S. et al. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends Neurosci. 2008;31:234–42. https://doi.org/10.1016/j.tins.2008.02.005.
Briend F, Nelson EA, Maximo O, Armstrong WP, Kraguljac NV, Lahti AC. Hippocampal glutamate and hippocampus subfield volumes in antipsychotic-naive first episode psychosis subjects and relationships to duration of untreated psychosis. Transl Psychiatry 2020;10. https://doi.org/10.1038/s41398-020-0812-z.
Tamminga CA, Stan AD, Wagner AD. The hippocampal formation in schizophrenia. Am J Psychiatry. 2010;167:1178–93. https://doi.org/10.1176/appi.ajp.2010.09081187.
Lieberman JA, Girgis RR, Brucato G, Moore H, Provenzano F, Kegeles L. et al. Hippocampal dysfunction in the pathophysiology of schizophrenia: a selective review and hypothesis for early detection and intervention. Mol Psychiatry. 2018;23:1764–72. https://doi.org/10.1038/mp.2017.249.
Roiser JP, Howes OD, Chaddock CA, Joyce EM, McGuire P. Neural and behavioral correlates of aberrant salience in individuals at risk for psychosis. Schizophr Bull. 2013;39:1328–36. https://doi.org/10.1093/schbul/sbs147.
Wolthusen RPF, Coombs G, Boeke EA, Ehrlich S, DeCross SN, Nasr S. et al. Correlation Between Levels of Delusional Beliefs and Perfusion of the Hippocampus and an Associated Network in a Non–Help-Seeking Population. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018;3:178–86. https://doi.org/10.1016/j.bpsc.2017.06.007.
Makowski C, Bodnar M, Shenker JJ, Malla AK, Joober R, Chakravarty MM, et al. Linking persistent negative symptoms to amygdala–hippocampus structure in first-episode psychosis. Transl Psychiatry 2017;7. https://doi.org/10.1038/tp.2017.168.
Achim AM, Lepage M. Episodic memory-related activation in schizophrenia: Meta-analysis. Br J Psychiatry. 2005;187:500–9. https://doi.org/10.1192/bjp.187.6.500.
Ranganath C, Minzenberg MJ, Ragland JD. The Cognitive Neuroscience of Memory Function and Dysfunction in Schizophrenia. Biol Psychiatry. 2008;64:18–25. https://doi.org/10.1016/j.biopsych.2008.04.011.
Guo JY, Ragland JD, Carter CS. Memory and cognition in schizophrenia. Mol Psychiatry. 2019;24:633–42. https://doi.org/10.1038/s41380-018-0231-1.
Heckers S, Rauch SL, Goff D, Savage CR, Schacter DL, Fischman AJ. et al. Impaired recruitment of the hippocampus during conscious recollection in schizophrenia. Nat Neurosci. 1998. 10.1038/1137.
McHugo M, Talati P, Armstrong K, Vandekar SN, Blackford JU, Woodward ND. et al. Hyperactivity and reduced activation of anterior hippocampus in early psychosis. Am J Psychiatry. 2019;176:1030–8. https://doi.org/10.1176/appi.ajp.2019.19020151.
Tregellas JR. Neuroimaging biomarkers for early drug development in schizophrenia. Biol Psychiatry. 2014;76:111–9. https://doi.org/10.1016/j.biopsych.2013.08.025.
Löscher W, Gillard M, Sands ZA, Kaminski RM, Klitgaard H. Synaptic Vesicle Glycoprotein 2A Ligands in the Treatment of Epilepsy and Beyond. CNS Drugs. 2016;30:1055–77. https://doi.org/10.1007/S40263-016-0384-X.
Lynch BA, Lambeng N, Nocka K, Kensel-Hammes P, Bajjalieh SM, Matagne A. et al. The synaptic vesicle is the protein SV2A is the binding site for the antiepileptic drug levetiracetam. Proc Natl Acad Sci USA. 2004;101:9861–6. https://doi.org/10.1073/pnas.0308208101.
Koh MT, Shao Y, Rosenzweig-Lipson S, Gallagher M. Treatment with levetiracetam improves cognition in a ketamine rat model of schizophrenia. Schizophr Res. 2018;193:119–25. https://doi.org/10.1016/j.schres.2017.06.027.
Cavichioli AM, Santos-Silva T, Grace AA, Guimarães FS, Gomes FV. Levetiracetam Attenuates Adolescent Stress-induced Behavioral and Electrophysiological Changes Associated With Schizophrenia in Adult Rats. Schizophr Bull 2022. https://doi.org/10.1093/schbul/sbac106.
Behdani F, Hassanzadeh B, Eslamzadeh M, Moradi M, Hebrani P, Dadgarmoghaddam M. et al. Can levetiracetam improve clinical symptoms in schizophrenic patients? A randomized placebo-controlled clinical trial. Int Clin Psychopharmacol. 2022;37:159–65. https://doi.org/10.1097/YIC.0000000000000405.
McHugo M, Rogers BP, Avery SN, Armstrong K, Blackford JU, Vandekar SN. et al. Increased amplitude of hippocampal low frequency fluctuations in early psychosis: A two-year follow-up study. Schizophr Res. 2022;241:260–6. https://doi.org/10.1016/J.SCHRES.2022.02.003.
Mathalon DH, Sohal VS. Neural Oscillations and Synchrony in Brain Dysfunction and Neuropsychiatric Disorders: It’s About Time. JAMA Psychiatry. 2015;72:840–4. https://doi.org/10.1001/JAMAPSYCHIATRY.2015.0483.
Niessing J, Ebisch B, Schmidt KE, Niessing M, Singer W, Galuske RAW. Neuroscience: Hemodynamic signals correlate tightly with synchronized gamma oscillations. Science. 2005;309:948–51. https://doi.org/10.1126/SCIENCE.1110948/SUPPL_FILE/NIESSING.SOM.PDF.
Hare SM, Law AS, Ford JM, Mathalon DH, Ahmadi A, Damaraju E. et al. Disrupted Network Cross Talk, Hippocampal Dysfunction and Hallucinations in Schizophrenia. Schizophr Res. 2018;199:226 https://doi.org/10.1016/J.SCHRES.2018.03.004.
McHugo M, Rogers BP, Talati P, Woodward ND, Heckers S. Increased amplitude of low frequency fluctuations but normal hippocampal-default mode network connectivity in schizophrenia. Front Psychiatry. 2015;6:92 https://doi.org/10.3389/FPSYT.2015.00092/BIBTEX.
Tang Y, Zhou Q, Chang M, Chekroud A, Gueorguieva R, Jiang X. et al. Altered functional connectivity and low-frequency signal fluctuations in early psychosis and genetic high risk. Schizophr Res. 2019;210:172–9. https://doi.org/10.1016/J.SCHRES.2018.12.041.
Turner JA, Damaraju E, Van Erp TGM, Mathalon DH, Ford JM, Voyvodic J. et al. A multi-site resting state fMRI study on the amplitude of low frequency fluctuations in schizophrenia. Front Neurosci. 2013;7:47140 https://doi.org/10.3389/FNINS.2013.00137/BIBTEX.
Hoptman MJ, Zuo XN, Butler PD, Javitt DC, D’Angelo D, Mauro CJ. et al. Amplitude of low-frequency oscillations in schizophrenia: A resting state fMRI study. Schizophr Res. 2010;117:13–20. https://doi.org/10.1016/J.SCHRES.2009.09.030.
Zou QH, Zhu CZ, Yang Y, Zuo XN, Long XY, Cao QJ. et al. An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: Fractional ALFF. J Neurosci Methods. 2008;172:137–41. https://doi.org/10.1016/J.JNEUMETH.2008.04.012.
Borogovac A, Asllani I. Arterial spin labeling (ASL) fMRI: Advantages, theoretical constrains and experimental challenges in neurosciences. Int J Biomed Imaging 2012;2012. https://doi.org/10.1155/2012/818456.
Petcharunpaisan S, Ramalho J, Castillo M. Arterial spin labeling in neuroimaging. World J Radiol. 2010;2:384 https://doi.org/10.4329/WJR.V2.I10.384.
Scheef L, Manka C, Daamen M, Kühn KU, Maier W, Schild HH. et al. Resting-state perfusion in nonmedicated schizophrenic patients: A continuous arterial spin-labeling 3.0-T MR study. Radiology. 2010;256:253–60. https://doi.org/10.1148/radiol.10091224.
Pinkham A, Loughead J, Ruparel K, Wu WC, Overton E, Gur R. et al. Resting quantitative cerebral blood flow in schizophrenia measured by pulsed arterial spin labeling perfusion MRI. Psychiatry Res Neuroimaging. 2011;194:64–72. https://doi.org/10.1016/j.pscychresns.2011.06.013.
Walther S, Federspiel A, Horn H, Razavi N, Wiest R, Dierks T. et al. Resting state cerebral blood flow and objective motor activity reveal basal ganglia dysfunction in schizophrenia. Psychiatry Res Neuroimaging. 2011;192:117–24. https://doi.org/10.1016/j.pscychresns.2010.12.002.
Kindler J, Jann K, Homan P, Hauf M, Walther S, Strik W. et al. Static and dynamic characteristics of cerebral blood flow during the resting state in schizophrenia. Schizophr Bull. 2015;41:163–70. https://doi.org/10.1093/schbul/sbt180.
Ota M, Ishikawa M, Sato N, Okazaki M, Maikusa N, Hori H, et al. Pseudo-continuous arterial spin labeling MRI study of schizophrenic patients. Schizophr Res. 2014;154:113–8. https://doi.org/10.1016/j.schres.2014.01.035.
Selvaggi P, Jauhar S, Kotoula V, Pepper F, Veronese M, Santangelo B, et al. Reduced cortical cerebral blood flow in antipsychotic-free first-episode psychosis and relationship to treatment response. Psychol Med. 2022:1–11. https://doi.org/10.1017/S0033291722002288.
Lahti AC, Holcomb HH, Weiler MA, Medoff DR, Tamminga CA. Functional effects of antipsychotic drugs: comparing clozapine with haloperidol. Biol Psychiatry. 2003;53:601–8. https://doi.org/10.1016/S0006-3223(02)01602-5.
Medoff DR, Holcomb HH, Lahti AC, Tamminga CA. Probing the human hippocampus using rCBF: Contrasts in schizophrenia. Hippocampus. 2001;11:543–50. https://doi.org/10.1002/HIPO.1070.
Alsop DC, Detre JA, Golay X, Günther M, Hendrikse J, Hernandez-Garcia L. et al. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn Reson Med. 2015;73:102–16. https://doi.org/10.1002/mrm.25197.
Seldin K, Armstrong K, Schiff ML, Heckers S. Reducing the Diagnostic Heterogeneity of Schizoaffective Disorder. Front Psychiatry 2017;8. https://doi.org/10.3389/FPSYT.2017.00018.
Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis: I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–94. https://doi.org/10.1006/nimg.1998.0395.
Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C. et al. Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–55. https://doi.org/10.1016/S0896-6273(02)00569-X.
Iglesias JE, Augustinack JC, Nguyen K, Player CM, Player A, Wright M. et al. A computational atlas of the hippocampal formation using ex vivo, ultra-high resolution MRI: Application to adaptive segmentation of in vivo MRI. Neuroimage. 2015;115:117–37. https://doi.org/10.1016/j.neuroimage.2015.04.042.
Woolard AA, Heckers S. Anatomical and functional correlates of human hippocampal volume asymmetry. Psychiatry Res Neuroimaging. 2012;201:48–53. https://doi.org/10.1016/j.pscychresns.2011.07.016.
Taylor PA, Saad ZS. FATCAT: (an efficient) Functional and Tractographic Connectivity Analysis Toolbox. Brain Connect. 2013;3:523–35. https://doi.org/10.1089/BRAIN.2013.0154.
Buxton RB, Frank LR, Wong EC, Siewert B, Warach S, Edelman RR. A general kinetic model for quantitative perfusion imaging with arterial spin labeling. Magn Reson Med. 1998. https://doi.org/10.1002/mrm.1910400308.
McHugo M, Avery S, Armstrong K, Rogers BP, Vandekar SN, Woodward ND, et al. Anterior hippocampal dysfunction in early psychosis: a 2-year follow-up study. Psychol Med. 2021:1–10. https://doi.org/10.1017/S0033291721001318.
Bates D, Machler M, Bolker B, Walker S. Fitting Linear Mixed-Effects Models Using lme4. J Stat Softw. 2015;67:1–48.
Fox J, Weisberg S. Package ‘car’. Companion to Applied Regression, Second Edition. 2011.
Kindler J, Schultze-Lutter F, Hauf M, Dierks T, Federspiel A, Walther S, et al. Increased Striatal and Reduced Prefrontal Cerebral Blood Flow in Clinical High Risk for Psychosis. Schizophr Bull. 2018;44:182 https://doi.org/10.1093/SCHBUL/SBX070.
Oliveira ÍAF, Guimarães TM, Souza RM, dos Santos AC, Machado-de-Sousa JP, Hallak JEC, et al. Brain functional and perfusional alterations in schizophrenia: an arterial spin labeling study. Psychiatry Res Neuroimaging. 2018;272:71–8. https://doi.org/10.1016/J.PSCYCHRESNS.2017.12.001.
Allen P, Chaddock CA, Egerton A, Howes OD, Bonoldi I, Zelaya F, et al. Resting hyperperfusion of the hippocampus, midbrain, and basal ganglia in people at high risk for psychosis. Am J Psychiatry. 2016;173:392–9. https://doi.org/10.1176/APPI.AJP.2015.15040485/ASSET/IMAGES/LARGE/APPI.AJP.2015.15040485F2.JPEG.
Allen P, Azis M, Modinos G, Bossong MG, Bonoldi I, Samson C, et al. Increased Resting Hippocampal and Basal Ganglia Perfusion in People at Ultra High Risk for Psychosis: Replication in a Second Cohort. Schizophr Bull. 2018;44:1323. https://doi.org/10.1093/SCHBUL/SBX169.
Handley R, Zelaya FO, Reinders AATS, Marques TR, Mehta MA, O’Gorman R, et al. Acute effects of single‐dose aripiprazole and haloperidol on resting cerebral blood flow (rCBF) in the human brain. Hum Brain Mapp. 2013;34:272. https://doi.org/10.1002/HBM.21436.
Ragland JD, Layher E, Hannula DE, Niendam TA, Lesh TA, Solomon M, et al. Impact of schizophrenia on anterior and posterior hippocampus during memory for complex scenes. Neuroimage Clin. 2017;13:82–8. https://doi.org/10.1016/J.NICL.2016.11.017.
Kelly AMC, Garavan H. Human functional neuroimaging of brain changes associated with practice. Cereb Cortex. 2005;15:1089–102. https://doi.org/10.1093/CERCOR/BHI005.
Onwordi EC, Halff EF, Whitehurst T, Mansur A, Cotel MC, Wells L, et al. Synaptic density marker SV2A is reduced in schizophrenia patients and unaffected by antipsychotics in rats. Nat Commun. 2020. https://doi.org/10.1038/s41467-019-14122-0.
Radhakrishnan R, Skosnik PD, Ranganathan M, Naganawa M, Toyonaga T, Finnema S, et al. In vivo evidence of lower synaptic vesicle density in schizophrenia. Mol Psychiatry. 2021;26:7690–8. https://doi.org/10.1038/S41380-021-01184-0.
Howes OD, Cummings C, Chapman GE, Shatalina E. Neuroimaging in schizophrenia: an overview of findings and their implications for synaptic changes. Neuropsychopharmacology. 2022. https://doi.org/10.1038/s41386-022-01426-x.
Onwordi EC, Whitehurst T, Shatalina E, Mansur A, Arumuham A, Osugo M, et al. Synaptic Terminal Density Early in the Course of Schizophrenia: an in vivo UCB-J Positron Emission Tomographic Imaging Study of Synaptic Vesicle Glycoprotein 2A (SV2A). Biol Psychiatry. 2023. https://doi.org/10.1016/J.BIOPSYCH.2023.05.022.
Yoon JH, Zhang Z, Mormino E, Davidzon G, Minzenberg MJ, Ballon J, et al. Reductions in synaptic marker SV2A in early-course Schizophrenia. J Psychiatr Res. 2023;161:213–7. https://doi.org/10.1016/J.JPSYCHIRES.2023.02.026.
Bakker A, Krauss GL, Albert MS, Speck CL, Jones LR, Stark CE, et al. Reduction of Hippocampal Hyperactivity Improves Cognition in Amnestic Mild Cognitive Impairment. Neuron. 2012;74:467–74. https://doi.org/10.1016/j.neuron.2012.03.023.
Bakker A, Albert MS, Krauss G, Speck CL, Gallagher M. Response of the medial temporal lobe network in amnestic mild cognitive impairment to therapeutic intervention assessed by fMRI and memory task performance. Neuroimage Clin. 2015;7:688–98. https://doi.org/10.1016/j.nicl.2015.02.009.
Lyseng-Williamson KA. Levetiracetam: A review of its use in epilepsy. Drugs. 2011;71:489–514. https://doi.org/10.2165/11204490-000000000-00000.
Smucny J, Olincy A, Rojas DC, Tregellas JR. Neuronal effects of nicotine during auditory selective attention in schizophrenia. Hum Brain Mapp. 2016;37:410–21. https://doi.org/10.1002/hbm.23040.
Kätzel D, Wolff AR, Bygrave AM, Bannerman DM. Hippocampal Hyperactivity as a Druggable Circuit-Level Origin of Aberrant Salience in Schizophrenia. Front Pharmacol 2020;11. https://doi.org/10.3389/FPHAR.2020.486811.
Funding
Research reported in this publication was supported by the Charlotte and Donald Test Fund, the Vanderbilt Psychiatric Genotype/Phenotype Project, the National Institute of Mental Health (NIMH) grants R01-MH70560 (SH) and F30-MH125507 (MJR), the National Institute of General Medical Sciences (NIGMS) grant T32-GM007347, and the Vanderbilt Institute for Clinical and Translational Research (through grant UL1-TR000445 from the National Center for Research Resources/NIH).
Author information
Authors and Affiliations
Contributions
Study concept, design, and acquisition of funding: MJR and SH. Participant recruitment: MJR, KA, and SH. Acquisition and processing of data: MJR, MM, BR, MD, and SH. Statistical analyses and interpretation of data: MJR, MM, SA, MD, SH. Drafting of manuscript: MJR and SH. Critical revision of manuscript: MJR, MM, BR, KA, SA, MD, SH. Study supervision: SH. All authors read and approve the manuscript and vouch for the adherence of the trial to the protocol, the accuracy of the data and analyses, and reporting of adverse events.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Roeske, M.J., McHugo, M., Rogers, B. et al. Modulation of hippocampal activity in schizophrenia with levetiracetam: a randomized, double-blind, cross-over, placebo-controlled trial. Neuropsychopharmacol. 49, 681–689 (2024). https://doi.org/10.1038/s41386-023-01730-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-023-01730-0