Abstract
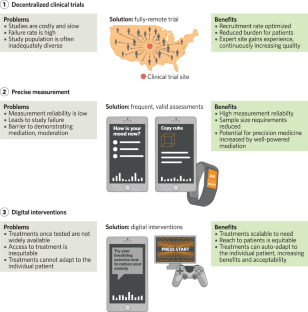
Mental health treatment advances - including neuropsychiatric medications and devices, psychotherapies, and cognitive treatments - lag behind other fields of clinical medicine such as cardiovascular care. One reason for this gap is the traditional techniques used in mental health clinical trials, which slow the pace of progress, produce inequities in care, and undermine precision medicine goals. Newer techniques and methodologies, which we term digital and precision trials, offer solutions. These techniques consist of (1) decentralized (i.e., fully-remote) trials which improve the speed and quality of clinical trials and increase equity of access to research, (2) precision measurement which improves success rate and is essential for precision medicine, and (3) digital interventions, which offer increased reach of, and equity of access to, evidence-based treatments. These techniques and their rationales are described in detail, along with challenges and solutions for their utilization. We conclude with a vignette of a depression clinical trial using these techniques.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 13 print issues and online access
$259.00 per year
only $19.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Change history
02 October 2023
A Correction to this paper has been published: https://doi.org/10.1038/s41386-023-01746-6
References
Stein DJ, Shoptaw SJ, Vigo DV, Lund C, Cuijpers P, Bantjes J, et al. Psychiatric diagnosis and treatment in the 21st century: paradigm shifts versus incremental integration. World Psychiatry. 2022;21:393–414. https://doi.org/10.1002/wps.20998.
Rush AJ, Sackeim HA, Conway CR, Bunker MT, Steven DH, Koen D, et al. Clinical research challenges posed by difficult-to-treat depression. Psychol Med. 2022;52:419–32. https://doi.org/10.1017/S0033291721004943.
Harvey PD, Strassnig MT. Cognition and disability in schizophrenia: cognition-related skills deficits and decision-making challenges add to morbidity. World Psychiatry. 2019;18:165–7. https://doi.org/10.1002/wps.20647.
Ringel MS, Scannell JW, Baedeker M, Schulze U. Breaking Eroom’s Law. Nat Rev Drug Discov. 2020;19:833–4. https://doi.org/10.1038/d41573-020-00059-3.
Manchia M, Pisanu C, Squassina A, Carpiniello B. Challenges and Future Prospects of Precision Medicine in Psychiatry. Pharmgenomics Pers Med. 2020;13:127–40. https://doi.org/10.2147/PGPM.S198225.
ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2002;288:2981–97. https://doi.org/10.1001/jama.288.23.2981.
Gaynes BN, Warden D, Trivedi MH, Wisniewski SR, Fava M, Rush AJ. What did STAR*D teach us? Results from a large-scale, practical, clinical trial for patients with depression. Psychiatr Serv. 2009;60:1439–45. https://doi.org/10.1176/ps.2009.60.11.1439.
Freedland KE. Progress in health-related behavioral intervention research: Making it, measuring it, and meaning it. Health Psychol. 2022;41:1–12. https://doi.org/10.1037/hea0001160.
Flyvbjerg B. Make Megaprojects More Modular. Harvard Business Rev. 2021; 58–63. Available at SSRN: https://ssrn.com/abstract=39374652021.
Mofsen AM, Rodebaugh TL, Nicol GE, Depp CA, Miller JP, Lenze EJ. When All Else Fails, Listen to the Patient: A Viewpoint on the Use of Ecological Momentary Assessment in Clinical Trials. JMIR Ment Health. 2019;6:e11845. https://doi.org/10.2196/11845.
Trajković G, Starčević V, Latas M, Miomir L, Tanja I, Zoran B, et al. Reliability of the Hamilton Rating Scale for Depression: A meta-analysis over a period of 49 years. Psychiatry Res. 2011;189:1–9. https://doi.org/10.1016/j.psychres.2010.12.007.
Enkavi AZ, Eisenberg IW, Bissett PG, Mazza GL, MacKinnon DP, Marsch LA, et al. Large-scale analysis of test-retest reliabilities of self-regulation measures. Proc Natl Acad Sci USA 2019;116:5472–7. https://doi.org/10.1073/pnas.1818430116.
Herting MM, Gautam P, Chen Z, Mezher A, Vetter NC. Test-retest reliability of longitudinal task-based fMRI: Implications for developmental studies. Dev Cogn Neurosci. 2018;33:17–26. https://doi.org/10.1016/j.dcn.2017.07.001.
Holiga S, Sambataro F, Luzy C, Greig G, Sarkar N, Remco RJ, et al. Test-retest reliability of task-based and resting-state blood oxygen level dependence and cerebral blood flow measures. PLoS One. 2018;13:e0206583. https://doi.org/10.1371/journal.pone.0206583.
Rodebaugh TL, Scullin RB, Langer JK, Dixon DJ, Huppert JD, Bernstein A, et al. Unreliability as a threat to understanding psychopathology: The cautionary tale of attentional bias. J Abnorm Psychol. 2016;125:840–51. https://doi.org/10.1037/abn0000184.
Lyon AR, Brewer SK, Arean PA. Leveraging human-centered design to implement modern psychological science: Return on an early investment. Am Psychol. 2020;75:1067–79. https://doi.org/10.1037/amp0000652.
Lyon AR, Munson SA, Renn BN, Atkins DC, Pullmann MD, Emily F, et al. Use of Human-Centered Design to Improve Implementation of Evidence-Based Psychotherapies in Low-Resource Communities: Protocol for Studies Applying a Framework to Assess Usability. JMIR Res Protoc. 2019;8:e14990. https://doi.org/10.2196/14990.
Munson SA, Friedman EC, Osterhage K, Allred R, Pullmann MD, Arean PA, et al. Usability Issues in Evidence-Based Psychosocial Interventions and Implementation Strategies: Cross-project Analysis. J Med Internet Res. 2022;24:e37585. https://doi.org/10.2196/37585.
Forjuoh SN, Helduser JW, Bolin JN, Ory MG. Challenges Associated with Multi-institutional Multi-site Clinical Trial Collaborations: Lessons from a Diabetes Self-Management Interventions Study in Primary Care. J Clin Trials. 2015;5:219. https://oaktrust.library.tamu.edu/handle/1969.1/154772.
Greer TL, Walker R, Rethorst CD, Northup TF, Diane W, Horigian VE, et al. Identifying and responding to trial implementation challenges during multisite clinical trials. J Subst Abus Treat. 2020;112:63–72. https://doi.org/10.1016/j.jsat.2020.02.004.
Kraemer HC. Pitfalls of multisite randomized clinical trials of efficacy and effectiveness. Schizophr Bull. 2000;26:533–41. https://doi.org/10.1093/oxfordjournals.schbul.a033474.
National Academies of Sciences Engineering, and Medicine. Virtual Clinical Trials: Challenges and Opportunities: A Workshop. 2019. https://www.nationalacademies.org/our-work/virtual-clinical-trials-challenges-and-opportunities-a-workshop.
Anguera JA, Jordan JT, Castaneda D, Gazzaley A, Arean PA. Conducting a fully mobile and randomised clinical trial for depression: access, engagement and expense. BMJ Innov. 2016;2:14–21. https://doi.org/10.1136/bmjinnov-2015-000098.
Ahern KB, Lenze EJ. Mental Health Clinical Research Innovations during the COVID-19 Pandemic: The Future Is Now. Psychiatr Clin North Am. 2022;45:179–89. https://doi.org/10.1016/j.psc.2021.11.011.
Lenze EJ, Mattar C, Zorumski CF, Stevens A, Schweiger J, Nicol GE, et al. Fluvoxamine vs Placebo and Clinical Deterioration in Outpatients With Symptomatic COVID-19: A Randomized Clinical Trial. JAMA. 2020;324:2292–300. https://doi.org/10.1001/jama.2020.22760.
Naggie S, Boulware DR, Lindsell CJ, Stewart TG, Slandzicki AJ, Lim SC, et al. Effect of Higher-Dose Ivermectin for 6 Days vs Placebo on Time to Sustained Recovery in Outpatients With COVID-19: A Randomized Clinical Trial. JAMA. 2023; https://doi.org/10.1001/jama.2023.1650.
Bramante CT, Beckman KB, Mehta T, Karger AB, Odde DJ, Tignanelli CJ, et al. Metformin reduces SARS-CoV-2 in a Phase 3 Randomized Placebo Controlled Clinical Trial. medRxiv. 2023:2023–06. https://doi.org/10.1101/2023.06.06.23290989.
Boulware DR, Pullen MF, Bangdiwala AS, Pastick KA, Lofgren SM, Okafor EC, et al. A Randomized Trial of Hydroxychloroquine as Postexposure Prophylaxis for Covid-19. N Engl J Med. 2020;383:517–25. https://doi.org/10.1056/NEJMoa2016638.
Comtois KA, Mata-Greve F, Johnson M, Pullmann MD, Mosser B, Arean P. Effectiveness of Mental Health Apps for Distress During COVID-19 in US Unemployed and Essential Workers: Remote Pragmatic Randomized Clinical Trial. JMIR Mhealth Uhealth. 2022;10:e41689. https://doi.org/10.2196/41689.
Arean PA, Hallgren KA, Jordan JT, Gazzaley A, Atkins DC, Heagerty PJ, et al. The Use and Effectiveness of Mobile Apps for Depression: Results From a Fully Remote Clinical Trial. J Med Internet Res. 2016;18:e330. https://doi.org/10.2196/jmir.6482.
Pratap A, Homiar A, Waninger L, Herd C, Suver C, Volponi J, et al. Real-world behavioral dataset from two fully remote smartphone-based randomized clinical trials for depression. Sci Data. 2022;9:522. https://doi.org/10.1038/s41597-022-01633-7.
Pratap A, Neto EC, Snyder P, Stepnowsky C, Elhadad N, Grant D, et al. Indicators of retention in remote digital health studies: a cross-study evaluation of 100,000 participants. NPJ Digit Med. 2020;3:21. https://doi.org/10.1038/s41746-020-0224-8.
Pratap A, Renn BN, Volponi J, Mooney SD, Gazzaley A, Arean PA, et al. Using Mobile Apps to Assess and Treat Depression in Hispanic and Latino Populations: Fully Remote Randomized Clinical Trial. J Med Internet Res. 2018;20:e10130. https://doi.org/10.2196/10130.
Ainsworth NJ, Wright H, Tereshchenko K, Blumberger DM, Flint AJ, Lenze EJ, et al. Recruiting for a Randomized Clinical Trial for Late-Life Depression During COVID-19: Outcomes of Provider Referrals Versus Facebook Self-Referrals. Am J Geriatr Psychiatry. 2023; https://doi.org/10.1016/j.jagp.2023.01.021.
Askin S, Burkhalter D, Calado G, El Dakrouni S. Artificial Intelligence Applied to clinical trials: opportunities and challenges. Health Technol. 2023;13:203–13. https://doi.org/10.1007/s12553-023-00738-2.
Miller MI, Shih LC, Kolachalama VB. Machine Learning in Clinical Trials: A Primer with Applications to Neurology. Neurotherapeutics. 2023:1–15. https://doi.org/10.1007/s13311-023-01384-2.
Hardman TC, Aitchison R, Scaife R, Edwards J, Slater G. The future of clinical trials and drug development: 2050. Drugs Context. 2023;12. https://doi.org/10.7573/dic.2023-2-2.
O’Donnell N, Satherley R, Davey E, Bryan G. Fraudulent participants in qualitative child health research: identifying and reducing bot activity. BMJ 2023;108:415.
Teitcher JE, Bockting WO, Bauermeister JA, Hoefer CJ, Miner MH, Klitzman RL. Detecting, preventing, and responding to "fraudsters" in internet research: ethics and tradeoffs. J Law Med Ethics. Spring. 2015;43:116–33. https://doi.org/10.1111/jlme.12200.
Storozuk A, Ashley M, Delage V, Maloney EA. Got bots? Practical recommendations to protect online survey data from bot attacks. Quant Methods Psychol. 2020;16:472–81.
Levi R, Ridberg R, Akers M, Seligman H. Survey Fraud and the Integrity of Web-Based Survey Research. Am J Health Promot. 2022;36:18–20. https://doi.org/10.1177/08901171211037531.
Campbell CK, Ndukwe S, Dube K, Sauceda JA, Saberi P. Overcoming Challenges of Online Research: Measures to Ensure Enrollment of Eligible Participants. J Acquir Immune Defic Syndr. 2022;91:232–6. https://doi.org/10.1097/QAI.0000000000003035.
Quagan B, Woods SW, Powers AR. Navigating the Benefits and Pitfalls of Online Psychiatric Data Collection. JAMA Psychiatry. 2021;78:1185–6. https://doi.org/10.1001/jamapsychiatry.2021.2315.
Leiner DJ. Too Fast, too Straight, too Weird: Non-Reactive Indicators for Meaningless Data in Internet Surveys. Surv Res Methods. 2019;13:229–48.
Salinas MR. Are Your Participants Real? Dealing with Fraud in Recruiting Older Adults Online. West J Nurs Res. 2023;45:93–99. https://doi.org/10.1177/01939459221098468.
Griffith Fillipo IR, Pullmann MD, Hull TD, James Z, Jerilyn W, Boris L, et al. Participant retention in a fully remote trial of digital psychotherapy: Comparison of incentive types. Front Digit Health. 2022;4:963741. https://doi.org/10.3389/fdgth.2022.963741.
Nickels S, Edwards MD, Poole SF, Winter D, Gronsbell J, Bella R, et al. Toward a Mobile Platform for Real-world Digital Measurement of Depression: User-Centered Design, Data Quality, and Behavioral and Clinical Modeling. JMIR Ment Health. 2021;8:e27589. https://doi.org/10.2196/27589.
Scheuer L, Torous J. Usable Data Visualization for Digital Biomarkers: An Analysis of Usability, Data Sharing, and Clinician Contact. Digit Biomark. 2022;6:98–106. https://doi.org/10.1159/000525888.
Clay I, Peerenboom N, Connors DE, Bourke S, Keogh A, Wac K, et al. Reverse Engineering of Digital Measures: Inviting Patients to the Conversation. Digit Biomark. 2023;7:28–44. https://doi.org/10.1159/000530413.
Dworkin RH, Turk DC, Peirce-Sandner S, Burke LB, Farrar JT, Gilron I, et al. Considerations for improving assay sensitivity in chronic pain clinical trials: IMMPACT recommendations. Pain. 2012;153:1148–58. https://doi.org/10.1016/j.pain.2012.03.003.
Kobak KA, Kane JM, Thase ME, Nierenberg AA. Why Do Clinical Trials Fail?: The Problem of Measurement Error in Clinical Trials: Time to Test New Paradigms? J Clin Psychopharmacol. 2007;27:1–5.
Khan A, Mar KF, Brown WA. The conundrum of depression clinical trials: one size does not fit all. Int Clin Psychopharmacol. 2018;33:239–48. https://doi.org/10.1097/YIC.0000000000000229.
Rutherford BR, Roose SP. A model of placebo response in antidepressant clinical trials. Am J Psychiatry. 2013;170:723–33. https://doi.org/10.1176/appi.ajp.2012.12040474.
Lenze EJ, Nicol GE, Barbour DL, Kannampallil T, Wong AWK, Piccirillo J, et al. Precision clinical trials: a framework for getting to precision medicine for neurobehavioural disorders. J Psychiatry Neurosci. 2021;46:E97–E110. https://doi.org/10.1503/jpn.200042.
Schiele MA, Zwanzger P, Schwarte K, Arolt V, Baune BT, Domschke K. Serotonin Transporter Gene Promoter Hypomethylation as a Predictor of Antidepressant Treatment Response in Major Depression: A Replication Study. Int J Neuropsychopharmacol. 2020;24:191–9. https://doi.org/10.1093/ijnp/pyaa081.
Stein K, Maruf AAL, Müller DJ, Bishop JR, Bousman CA. Serotonin Transporter Genetic Variation and Antidepressant Response and Tolerability: A Systematic Review and Meta-Analysis. J Personalized Med. 2021;11:1334.
Klasnja P, Hekler EB, Shiffman S, Boruvka A, Almirall D, Tewari A, et al. Microrandomized trials: An experimental design for developing just-in-time adaptive interventions. Health Psychol. 2015;34S:1220–8. https://doi.org/10.1037/hea0000305.
Almirall D, Nahum-Shani I, Sherwood NE, Murphy SA. Introduction to SMART designs for the development of adaptive interventions: with application to weight loss research. Transl Behav Med. 2014;4:260–74. https://doi.org/10.1007/s13142-014-0265-0.
Hoyle RH, Robinson JC. Mediated and Moderated Effects in Social Psychological Research: Measurement, Design, and analysis Issues. In: Sansone C, Morf CC, Panter AT, eds. The Sage Handbook of Methods in Social Psychology. SAGE; 2004:chap 10.
Michon KJ, Khammash D, Simmonite M, Hamlin AM, Polk TA. Person-specific and precision neuroimaging: Current methods and future directions. NeuroImage. 2022;263:119589. https://doi.org/10.1016/j.neuroimage.2022.119589.
Shiffman S, Stone AA, Hufford MR. Ecological momentary assessment. Annu Rev Clin Psychol. 2008;4:1–32.
Zulueta J, Piscitello A, Rasic M, Easter R, Babu P, Langenecker SA, et al. Predicting Mood Disturbance Severity with Mobile Phone Keystroke Metadata: A BiAffect Digital Phenotyping Study. J Med Internet Res. 2018;20:e241. https://doi.org/10.2196/jmir.9775.
Torous J, Kiang MV, Lorme J, Onnela JP. New Tools for New Research in Psychiatry: A Scalable and Customizable Platform to Empower Data Driven Smartphone Research. JMIR Ment Health. 2016;3:e16. https://doi.org/10.2196/mental.5165.
Ebner-Priemer UW, Trull TJ. Ecological momentary assessment of mood disorders and mood dysregulation. Psychol Assess. 2009;21:463–75. https://doi.org/10.1037/a0017075.
Moore RC, Ackerman RA, Russell MT, Campbell LM, Depp CA, Harvey PD, et al. Feasibility and validity of ecological momentary cognitive testing among older adults with mild cognitive impairment. Front Digit Health. 2022;4:946685. https://doi.org/10.3389/fdgth.2022.946685.
Moore RC, Depp CA, Wetherell JL, Lenze EJ. Ecological momentary assessment versus standard assessment instruments for measuring mindfulness, depressed mood, and anxiety among older adults. J Psychiatr Res. 2016;75:116–23. https://doi.org/10.1016/j.jpsychires.2016.01.011.
Nicosia J, Aschenbrenner AJ, Balota DA, Sliwinski MJ, Marisol T, Adams S, et al. Unsupervised high-frequency smartphone-based cognitive assessments are reliable, valid, and feasible in older adults at risk for Alzheimer’s disease. J Int Neuropsychol Soc. 2022:1–13. https://doi.org/10.1017/S135561772200042X.
Moore RC, Swendsen J, Depp CA. Applications for self-administered mobile cognitive assessments in clinical research: A systematic review. Int J Methods Psychiatr Res. 2017;26. https://doi.org/10.1002/mpr.1562.
Alva S, Brazg R, Castorino K, Kipnes M, Liljenquist DR, Liu H. Accuracy of the Third Generation of a 14-Day Continuous Glucose Monitoring System. Diabetes Ther. 2023; https://doi.org/10.1007/s13300-023-01385-6.
Badal VD, Parrish EM, Holden JL, Depp CA, Granholm E. Dynamic contextual influences on social motivation and behavior in schizophrenia: a case-control network analysis. NPJ Schizophr. 2021;7:62. https://doi.org/10.1038/s41537-021-00189-6.
Bagby RM, Ryder AG, Schuller DR, Marshall MB. The Hamilton Depression Rating Scale: has the gold standard become a lead weight? Am J Psychiatry. 2004;161:2163–77.
Brady LS, Larrauri CA, Committee ASS. Accelerating Medicines Partnership® Schizophrenia (AMP®SCZ): developing tools to enable early intervention in the psychosis high risk state. World Psychiatry. 2023;22:42–3. https://doi.org/10.1002/wps.21038.
Coats AJ, Radaelli A, Clark SJ, Conway J, Sleight P. The influence of ambulatory blood pressure monitoring on the design and interpretation of trials in hypertension. J Hypertens. 1992;10:385–91. https://doi.org/10.1097/00004872-199204000-00011.
Oreel TH, Delespaul P, Hartog ID, Henriques JPS, Netjes JE, Vonk ABA, et al. Ecological momentary assessment versus retrospective assessment for measuring change in health-related quality of life following cardiac intervention. J Patient-Rep. Outcomes. 2020;4:98. https://doi.org/10.1186/s41687-020-00261-2.
Andrewes HE, Hulbert C, Cotton SM, Betts J, Chanen AM. An ecological momentary assessment investigation of complex and conflicting emotions in youth with borderline personality disorder. Psychiatry Res. 2017;252:102–10. https://doi.org/10.1016/j.psychres.2017.01.100.
Pecina M, Chen J, Karp JF, Dombrovski AY. Dynamic Feedback Between Antidepressant Placebo Expectancies and Mood. JAMA Psychiatry. 2023; https://doi.org/10.1001/jamapsychiatry.2023.0010.
Yaden DB, Potash JB, Griffiths RR. Preparing for the Bursting of the Psychedelic Hype Bubble. JAMA Psychiatry. 2022;79:943–4. https://doi.org/10.1001/jamapsychiatry.2022.2546.
Trivedi M, Carpenter L, Thase M. Clinical Outcome Assessments (COA) Qualification Program DDT COA #000008: Symptoms of Major Depressive Disorder Scale (SMDDS) Full Qualification Package. 2018. https://www.fda.gov/drugs/clinical-outcome-assessment-coa-qualification-program/ddt-coa-000008-symptoms-major-depressive-disorder-scale-smdds.
White House Report on Mental Health Research Priorities (2023). https://www.whitehouse.gov/ostp/news-updates/2023/02/07/white-house-report-on-mental-health-research-priorities/.
Mental Health By the Numbers. NAMI. 2023. https://www.nami.org/mhstats.
Behavioral Health Workforce Projections. HRSA Health Workforce https://bhw.hrsa.gov/data-research/projecting-health-workforce-supply-demand/behavioral-health.
Goldberg SB, Lam SU, Simonsson O, Torous J, Sun S. Mobile phone-based interventions for mental health: A systematic meta-review of 14 meta-analyses of randomized controlled trials. PLOS Digit Health. 2022;1. https://doi.org/10.1371/journal.pdig.0000002.
Freedland KE, Mohr DC, Davidson KW, Schwartz JE. Usual and unusual care: existing practice control groups in randomized controlled trials of behavioral interventions. Rev Psychosom Med.2011;73:323–35. https://doi.org/10.1097/PSY.0b013e318218e1fb.
Lyles CR, Wachter RM, Sarkar U. Focusing on Digital Health Equity. JAMA. 2021;326:1795–6. https://doi.org/10.1001/jama.2021.18459.
Mishori R, Antono B. Telehealth, Rural America, and the Digital Divide. J Ambulatory Care Manag. 2020;43:319–22.
Sosa Diaz MJ. Emergency Remote Education, Family Support and the Digital Divide in the Context of the COVID-19 Lockdown. Int J Environ Res Public Health. 2021;18. https://doi.org/10.3390/ijerph18157956.
Killsback LK. A nation of families: traditional indigenous kinship, the foundation for Cheyenne sovereignty. AlterNative: Int J Indigenous Peoples. 2019;15:34–43. https://doi.org/10.1177/1177180118822833.
ADA Archive, Department of Justice Civil Rights Division. 2023. https://archive.ada.gov/access-technology/index.html.
How to Check for App Accessibility? Perkins School for the Blind. 2023. https://www.perkins.org/resource/how-check-app-accessibility/.
Martinez-Alcala CI, Rosales-Lagarde A, Perez-Perez Y, Lopez-Noguerola JS, Bautista-Diaz M, Agis-Juarez RA. The Effects of Covid-19 on the Digital Literacy of the Elderly: Norms for Digital Inclusion. Front Educ. 2021;6:1–19.
Grossman JT, Frumkin MR, Rodebaugh TL, Lenze EJ. mHealth Assessment and Intervention of Depression and Anxiety in Older Adults. Harv Rev Psychiatry. 2020;28:203–14. https://doi.org/10.1097/HRP.0000000000000255.
Bach AJ, Wolfson T, Crowell JK. Poverty, Literacy, and Social Transformation: An Interdisciplinary Exploration of the Digital Divide. J Media Lit Educ. 2018;10:22–41.
Lee J, Lee EH, Chae D. eHealth Literacy Instruments: Systematic Review of Measurement Properties. J Med Internet Res. 2021;23:e30644. https://doi.org/10.2196/30644.
Oh SS, Kim KA, Kim M, Oh J, Chu SH, Choi J. Measurement of Digital Literacy Among Older Adults: Systematic Review. J Med Internet Res. 2021;23:e26145. https://doi.org/10.2196/26145.
Yoon J, Lee M, Ahn JS, Oh D, Shin S-Y, Chang YJ, et al. Development and Validation of Digital Health Technology Literacy Assessment Questionnaire. J Med Syst. 2022;46:13. https://doi.org/10.1007/s10916-022-01800-8.
Rivadeneira MF, Miranda-Velasco MJ, Arroyo HV, Caicedo-Gallardo JD, Salvador-Pinos C. Digital Health Literacy Related to COVID-19: Validation and Implementation of a Questionnaire in Hispanic University Students. Int J Environ Res Public Health. 2022;19. https://doi.org/10.3390/ijerph19074092.
U.S. Food & Drug Administration. Digital Health Technologies for Drug Development: Demonstration Projects. 2023. https://www.fda.gov/science-research/science-and-research-special-topics/digital-health-technologies-drug-development-demonstration-projects.
U.S. Food & Drug Administration. The Software Precertification (Pre-Cert) Pilot Program: Tailored Total Product Lifecycle Approaches and Key Findings. 2022. https://www.fda.gov/media/161815/download.
Zarate D, Stavropoulos V, Ball M, de Sena Collier G, Jacobson NC. Exploring the digital footprint of depression: a PRISMA systematic literature review of the empirical evidence. BMC Psychiatry. 2022;22:421. https://doi.org/10.1186/s12888-022-04013-y.
Ortiz A, Maslej MM, Husain MI, Daskalakis ZJ, Mulsant BH. Apps and gaps in bipolar disorder: A systematic review on electronic monitoring for episode prediction. J Affect Disord. 2021;295:1190–200. https://doi.org/10.1016/j.jad.2021.08.140.
Benoit J, Onyeaka H, Keshavan M, Torous J. Systematic Review of Digital Phenotyping and Machine Learning in Psychosis Spectrum Illnesses. Harv Rev Psychiatry. 2020;28:296–304. https://doi.org/10.1097/HRP.0000000000000268.
Matcham F, Leightley D, Siddi S, Lamers F, White KM, Annas P, et al. Remote Assessment of Disease and Relapse in Major Depressive Disorder (RADAR-MDD): recruitment, retention, and data availability in a longitudinal remote measurement study. BMC Psychiatry. 2022;22:136. https://doi.org/10.1186/s12888-022-03753-1.
Currey D, Torous J. Increasing the Value of Digital Phenotyping Through Reducing Missingness: A Retrospective Analysis. medRxiv. 2022. https://doi.org/10.1101/2022.05.17.22275182.
Torous LS. Usable Data Visualization for Digital Biomarkers: An Analysis of Usability, Data Sharing, and Clinician Contact.
Ghafur S, Van Dael J, Leis M, Darzi A, Sheikh A. Public perceptions on data sharing: key insights from the UK and the USA. Lancet Digit Health. 2020;2:e444–6. https://doi.org/10.1016/S2589-7500(20)30161-8.
Huberty J. Real Life Experiences as Head of Science. JMIR Ment Health. 2023;10:e43820. https://doi.org/10.2196/43820.
Kwon S, Firth J, Joshi D, Torous J. Accessibility and availability of smartphone apps for schizophrenia. Schizophrenia (Heidelb). 2022;8:98. https://doi.org/10.1038/s41537-022-00313-0.
Author information
Authors and Affiliations
Contributions
EL, JT, and PA all participated in developing the concept for the manuscript, writing the manuscript, and critically reviewing and editing it.
Corresponding author
Ethics declarations
Competing interests
EL: Consultant for Prodeo, Pritikin ICR, IngenioRx, Boehringer-Ingelheim, and Merck. Research funding from Janssen. Patent application pending for sigma-1 receptor agonists for COVID-19. JT: scientific advisory board of Precision Mental Wellness. PA: scientific advisory board of Headspace Health, Koa Health, and Chorus Sleep.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: in figure 1, the text boxes on the left inadvertently repeated the same information. The figure has now been replaced with an updated version.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lenze, E., Torous, J. & Arean, P. Digital and precision clinical trials: innovations for testing mental health medications, devices, and psychosocial treatments. Neuropsychopharmacol. 49, 205–214 (2024). https://doi.org/10.1038/s41386-023-01664-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-023-01664-7