Abstract
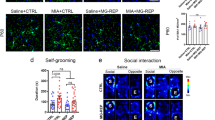
The peripartum period is accompanied by peripheral immune alterations to promote a successful pregnancy. We and others have also demonstrated significant neuroimmune changes that emerge during late pregnancy and persist postpartum, most prominently decreased microglia numbers within limbic brain regions. Here we hypothesized that microglial downregulation is important for the onset and display of maternal behavior. To test this, we recapitulated the peripartum neuroimmune profile by depleting microglia in non-mother (i.e., nulliparous) female rats who are typically not maternal but can be induced to behave maternally towards foster pups after repeated exposure, a process called maternal sensitization. BLZ945, a selective colony-stimulating factor 1 receptor (CSF1R) inhibitor, was administered systemically to nulliparous rats, which led to ~75% decrease in microglia number. BLZ- and vehicle-treated females then underwent maternal sensitization and tissue was stained for ∆fosB to examine activation across maternally relevant brain regions. We found BLZ-treated females with microglial depletion met criteria for displaying maternal behavior significantly sooner than vehicle-treated females and displayed increased pup-directed behaviors. Microglia depletion also reduced threat appraisal behavior in an open field test. Notably, nulliparous females with microglial depletion had decreased numbers of ∆fosB+ cells in the medial amygdala and periaqueductal gray, and increased numbers in the prefrontal cortex and somatosensory cortex, compared to vehicle. Our results demonstrate that microglia regulate maternal behavior in adult females, possibly by shifting patterns of activity in the maternal brain network.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 13 print issues and online access
$259.00 per year
only $19.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Sedgh G, Singh S, Hussain R. Intended and unintended pregnancies worldwide in 2012 and recent trends. Stud Fam Plann. 2014;45:301–14.
Orso R, Creutzberg KC, Wearick-Silva LE, Wendt Viola T, Tractenberg SG, Benetti F, et al. How early life stress impact maternal care: a systematic review of rodent studies. Front Behav Neurosci. 2019;13:197.
Champagne FA. Epigenetic mechanisms and the transgenerational effects of maternal care. Front Neuroendocrinol. 2008;29:386–97.
Brummelte S, Galea LAM. Postpartum depression: etiology, treatment and consequences for maternal care. Horm Behav. 2016;77:153–66.
Russell JA, Brunton PJ. The expectant brain: adapting for motherhood. Nat Rev Neurosci. 2008;9:11–25.
Numan M. The parental brain. Mechanisms, development and evolution. Oxford University Press: Oxford, England. 2020.
Numan M. Maternal behavior: neural circuits, stimulus valence, and motivational processes. Parent Sci Pract. 2012;12:105–14.
Stern JM. Maternal behavior: sensory, hormonal, and neural determinants. In: Brush FR, Levine S, editors. Psychoendocrinology. Academic Press: Harcourt Brace Jovanovich; New York, NY. 1989: 105–226.
Numan M, Stolzenberg DS. Medial preoptic area interactions with dopamine neural systems in the control of the onset and maintenance of maternal behavior in rats. Front Neuroendocrinol. 2008;30:46–64.
Napso T, Yong HEJ, Lopez-Tello J, Sferruzzi-Perri AN. The role of placental hormones in mediating maternal adaptations to support pregnancy and lactation. Front Physiol. 2018;9:1091.
Murrieta-Coxca J, Rodríguez-Martínez S, Cancino-Diaz ME, Markert UR, Favaro RR, Morales-Prieto DM. IL-36 cytokines: regulators of inflammatory responses and their emerging role in immunology of reproduction. Int J Mol Sci. 2019;20:1649.
Mor G, Cardenas I. The immune system in pregnancy: a unique complexity. Am J Reprod Immunol. 2010;63:425–33.
Dye C, Lenz KM, Leuner B. Immune system alterations and postpartum mental illness: evidence from basic and clinical research. Front Glob Women’s Health. 2022;2:758748.
Haim A, Julian D, Albin-Brooks C, Brothers HM, Lenz KM, Leuner B. A survey of neuroimmune changes in pregnant and postpartum female rats. Brain Behav Immun. 2016;59:67–78.
Posillico CK, Schwarz JM. An investigation into the effects of antenatal stressors on the postpartum neuroimmune profile and depressive-like behaviors. Behav Brain Res. 2016;298:218–28.
Kohl J, Dulac C. Neural control of parental behaviors. Curr Opin Neurobiol. 2018;49:116–22.
Green KN, Crapser JD, Hohsfield LA. To kill a microglia: a case for CSF1R inhibitors. Trends Immunol. 2020;41:771–84.
Elmore MRP, Najafi AR, Koike MA, Dagher NN, Spangenberg EE, Rice RA, et al. Colony-stimulating factor 1 receptor signaling is necessary for microglia viability, unmasking a microglia progenitor cell in the adult brain. Neuron. 2014;82:380–97.
Torres L, Danver J, Ji K, Miyauchi JT, Chen D, Anderson ME, et al. Dynamic microglial modulation of spatial learning and social behavior. Brain Behav Immun. 2016;55:6–16.
Champagne F, Diorio J, Sharma S, Meaney MJ. Naturally occurring variations in maternal behavior in the rat are associated with differences in estrogen-inducible central oxytocin receptors. Proc Natl Acad Sci USA. 2001;98:12736–41.
Fleming AS, Sarker J. Experience-hormone interactions and maternal behavior in rats. Physiol Behav. 1990;47:1165.
Leuner B, Shors TJ. Learning during motherhood: a resistance to stress. Horm Behav. 2006;50:38–51.
Li M, Fleming AS. The nucleus accumbens shell is critical for normal expression of pup-retrieval in postpartum female rats. Behav Brain Res. 2003;145:99.
Peña CJ, Neugut YD, Champagne FA. Developmental timing of the effects of maternal care on gene expression and epigenetic regulation of hormone receptor levels in female rats. Endocrinology. 2013;154:4340–51.
Ragan CM, Ahmed EI, Vitale EM, Linning-Duffy K, Miller-Smith SM, Maguire J, et al. Postpartum state, but not maternal caregiving or level of anxiety, increases medial prefrontal cortex GAD65 and vGAT in female rats. Front Glob Women’s Health. 2022;2:746518.
Rees SL, Panesar S, Steiner M, Fleming AS. The effects of adrenalectomy and corticosterone replacement on induction of maternal behavior in the virgin female rat. Horm Behav. 2006;49:337–45.
Pedersen CA, Prange AJ Jr. Induction of maternal behavior in virgin rats after intracerebroventricular administration of oxytocin. Proc Natl Acad Sci USA. 1979;76:6661–5.
Nestler EJ. ΔFosB: a transcriptional regulator of stress and antidepressant responses. Eur J Pharmacol. 2014;753:66–72.
Hagemeyer N, Hanft K-M, Akriditou M-A, Unger N, Park ES, Stanley ER, et al. Microglia contribute to normal myelinogenesis and to oligodendrocyte progenitor maintenance during adulthood. Acta Neuropathol. 2017;134:441–58.
Rosenblatt JS. Nonhormonal basis of maternal behavior in the rat. Science. 1967;156:1512–4.
Fleming AS, Vaccarino F, Luebke C. Amygdaloid inhibition of maternal behavior in the nulliparous female rat. Physiol Behav. 1980;25:731.
Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; Waltham, MA. 1982.
Salvatore M, Wiersielis KR, Luz S, Waxler DE, Bhatnagar S, Bangasser DA. Sex differences in circuits activated by corticotropin releasing factor. Horm Behav. 2018;97:145–53.
Stern JM. Maternal behavior priming in virgin and caesarean-delivered Long-Evans rats: effects of brief contact or continuous exteroceptive pup stimulation. Physiol Behav. 1983;31:757.
Fleming AS, Rosenblatt JS. Olfactory regulation of maternal behavior in rats: I. Effects of olfactory bulb removal in experienced and inexperienced lactating and cycling females. J Comp Physiol Psychol. 1974;86:221–32.
Lonstein JS. Regulation of anxiety during the postpartum period. Front Neuroendocrinol. 2007;28:115–41.
Fleming A, Vaccarino F, Tambosso L, Chee P. Vomeronasal and olfactory system modulation of maternal behavior in the rat. Science 1979;203:372–4.
Morgan HD, Fleming AS, Stern JM. Somatosensory control of the onset and retention of maternal responsiveness in primiparous Sprague Dawley rats. Physiol Behav. 1992;51:549.
Brecht M. Barrel cortex and whisker-mediated behaviors. Curr Opin Neurobiol. 2007;17:408–16.
Rosselet C, Zennou-Azogui Y, Xerri C. Nursing-induced somatosensory cortex plasticity: temporally decoupled changes in neuronal receptive field properties are accompanied by modifications in activity-dependent protein expression. J Neurosci. 2006;26:10667–76.
Erblich B, Zhu L, Etgen AM, Dobrenis K, Pollard JW. Absence of colony stimulation factor-1 receptor results in loss of microglia, disrupted brain development and olfactory deficits. PLoS ONE. 2011;6:e26317.
Wallace J, Lord J, Dissing-Olesen L, Stevens B, Murthy VN. Microglial depletion disrupts normal functional development of adult-born neurons in the olfactory bulb. eLife. 2020;9:e50531.
Champagne FA, Chretien P, Stevenson CW, Zhang TY, Gratton A, Meaney MJ. Variations in nucleus accumbens dopamine associated with individual differences in maternal behavior in the rat. J Neurosci. 2004;24:4113–23.
Rincon-Cortes M, Grace AA. Adaptations in reward-related behaviors and mesolimbic dopamine function during motherhood and the postpartum period. Front Neuroendocrinol. 2020;57:100839.
Olazabal DE, Pereira M, Uriarte N, Agrati D, Ferreira A, Fleming AS, et al. Flexibility and adaptation of the neural substrate that supports maternal behavior in mammals. Neurosci Biobehav Rev. 2013;37:1875–92.
Afonso VM, Sison M, Lovic V, Fleming AS. Medial prefrontal cortex lesions in the female rat affect sexual and maternal behavior and their sequential organization. Behav Neurosci. 2007;121:515–26.
Leuner B, Gould E. Dendritic growth in medial prefrontal cortex and cognitive flexibility are enhanced during the postpartum period. J Neurosci. 2010;30:13499–503.
Lovic V, Fleming AS. Artificially-reared female rats show reduced prepulse inhibition and deficits in the attentional set shifting task-reversal of effects with maternal-like licking stimulation. Behav Brain Res. 2004;148:209.
Eyo UB, Wu L-J. Bidirectional microglia-neuron communication in the healthy brain. Neural Plast. 2013;2013:456857.
Wu Y, Dissing-Olesen L, MacVicar BA, Stevens B. Microglia: dynamic mediators of synapse development and plasticity. Trend Immunol. 2015;36:605–13.
Nelson LH, Peketi P, Lenz KM. Microglia regulate cell genesis in a sex-dependent manner in the neonatal hippocampus. Neurosci. 2021;453:237–55.
Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, et al. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74:691–705.
Biber K, Vinet J, Boddeke HWGM. Neuron-microglia signaling: chemokines as versatile messengers. J Neuroimmunol. 2008;198:69–74.
Rice RA, Spangenberg EE, Yamate-Morgan H, Lee RJ, Arora RPS, Hernandez MX, et al. Elimination of microglia improves functional outcomes following extensive neuronal loss in the hippocampus. J Neurosci. 2015;35:9977–89.
Rasia-Filho AA, Fabian C, Rigoti KM, Achaval M. Influence of sex, estrous cycle and motherhood on dendritic spine density in the rat medial amygdala revealed by the golgi method. Neurosci. 2004;126:839–47.
Sheppard PAS, Choleris E, Galea LAM. Structural plasticity of the hippocampus in response to estrogens in female rodents. Mol Brain 2019;12:17.
Badimon A, Strasburger HJ, Ayata P, Chen X, Nair A, Ikegami A, et al. Negative feedback control of neuronal activity by microglia. Nature. 2020;586:417.
Lin SH, Kiyohara T, Sun B. Maternal behavior: activation of the central oxytocin receptor system in parturient rats? Neuroreport. 2003;14:1439–44.
Mathieson WB, Wilkinson M, Brown RE, Bond TLY, Taylor SW, Neumann PE. FOS and FOSB expression in the medial preoptic nucleus pars compacta of maternally active C57BL/6J and DBA/2J mice. Brain Res. 2002;952:170–5.
Nomaru H, Sakumi K, Katogi A, Ohnishi YN, Kajitani K, Tscuchimoto D, et al. Fosb gene products contribute to excitotoxic microglia activation by regulating the expression of complement C5a receptors in microglia. Glia. 2014;62:1284–98.
Patterson JR, Kim EJ, Goudreau JL, Lookingland KJ. FosB and ∆FosB expression in brain regions containing differentially susceptible dopamine neurons following acute neurotoxicant exposure. Brain Res. 2016;1649:53–66.
Brown JR, Ye H, Bronson RT, Dikkes P, Greenberg ME. A defect in nurturing in mice lacking the immediate early gene fosB. Cell. 1996;86:297–309.
Lei F, Cui N, Zhou C, Chodosh J, Vavvas DG, Paschailis EI. CSF1R inhibition by a small-molecule inhibitor is not microglia specific; affecting hematopoiesis and the function of macrophages. Proc Natl Acad Sci USA. 2020;117:23336–8.
Wohleb ES, Delpech J-C. Dynamic cross-talk between microglia and peripheral monocytes underlies stress-induced neuroinflammation and behavioral consequences. Prog Neuropsychopharmacol Biol Psychiatry. 2017;70:40–48.
Weber MD, Godbout JP, Sheridan JF. Repeated social defeat, neuroinflammation, and behavior: monocytes carry the signal. Neuropsychopharmacology. 2017;42:46–61.
Moore CL. Sex differences in urinary odors produced by young laboratory rats (Rattus norvegicus). J Comp Psychol. 1985;99:336–41.
Bowers JM, Perez-Pouchoulen M, Edwards NS, McCarthy MM. Foxp2 mediates sex differences in ultrasonic vocalization by rat pups and directs order of maternal retrieval. J Neurosci. 2013;33:3276–83.
Funding
National Institute of Mental Health grant (R21 MH117482-02) to BL and KML; National Science Foundation grant (2114381) to BL and KML; CND was supported by National Institute of Neurological Disorders and Stroke (T32NS105864).
Author information
Authors and Affiliations
Contributions
CND performed experiments, analyzed the data, and wrote and edited the manuscript. DF contributed to behavior analysis. BL and KML conceptualized and supervised the experiments and wrote and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dye, C.N., Franceschelli, D., Leuner, B. et al. Microglia depletion facilitates the display of maternal behavior and alters activation of the maternal brain network in nulliparous female rats. Neuropsychopharmacol. 48, 1869–1877 (2023). https://doi.org/10.1038/s41386-023-01624-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-023-01624-1
This article is cited by
-
The transition to motherhood: linking hormones, brain and behaviour
Nature Reviews Neuroscience (2023)