Abstract
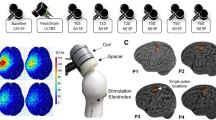
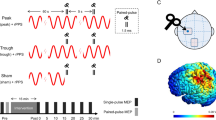
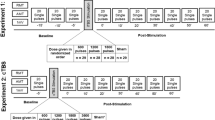
Repeated spaced TMS protocols, also termed accelerated TMS protocols, are of increasing therapeutic interest. The long-term potentiation (LTP)-like effects of repeated spaced intermittent theta-burst transcranial magnetic stimulation (iTBS) are presumed to be N-Methyl-D-Aspartate receptor (NMDA-R) dependent; however, this has not been tested. We tested whether the LTP-like effects of repeated spaced iTBS are influenced by low-dose D-Cycloserine (100 mg), an NMDA-R partial-agonist. We conducted a randomized, double-blind, placebo-controlled crossover trial in 20 healthy adults from August 2021-Feb 2022. Participants received repeated spaced iTBS, consisting of two iTBS sessions 60 minutes apart, to the primary motor cortex. The peak-to-peak amplitude of the motor evoked potentials (MEP) at 120% resting motor threshold (RMT) was measured after each iTBS. The TMS stimulus-response (TMS-SR; 100–150% RMT) was measured at baseline, +30 min, and +60 min after each iTBS. We found evidence for a significant Drug*iTBS effect in MEP amplitude, revealing that D-Cycloserine enhanced MEP amplitudes relative to the placebo. When examining TMS-SR, pairing iTBS with D-Cycloserine increased the TMS-SR slope relative to placebo after both iTBS tetani, and this was due to an increase in the upper bound of the TMS-SR. This indicates that LTP-like and metaplastic effects of repeated-spaced iTBS involve NMDA-R, as revealed by two measures of corticospinal excitability, and that low-dose D-Cycloserine facilitates the physiological effects of repeated spaced iTBS. However, extension of these findings to clinical populations and therapeutic protocols targeting non-motor regions of cortex requires empirical validation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 13 print issues and online access
$259.00 per year
only $19.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Data from this study is available upon reasonable request from the corresponding author.
References
Huang Y-Z, Lu M-K, Antal A, Classen J, Nitsche M, Ziemann U, et al. Plasticity induced by non-invasive transcranial brain stimulation: A position paper. Clin Neurophysiol. 2017;128:2318–29.
Goldsworthy MR, Pitcher JB, Ridding MC. Spaced noninvasive brain stimulation: Prospects for inducing long-lasting human cortical plasticity. Neurorehabilit Neural Repair. 2015;29:714–21.
Smolen P, Zhang Y, Byrne JH. The right time to learn: mechanisms and optimization of spaced learning. Nat Rev Neurosci. 2016;17:77–88.
Abraham WC, Huggett A. Induction and reversal of long-term potentiation by repeated high-frequency stimulation in rat hippocampal slices. Hippocampus 1997;7:137–45.
Kramár EA, Babayan AH, Gavin CF, Cox CD, Jafari M, Gall CM, et al. Synaptic evidence for the efficacy of spaced learning. Proc Natl Acad Sci USA. 2012;109:5121–6.
Lynch G, Kramár EA, Babayan AH, Rumbaugh G, Gall CM. Differences between synaptic plasticity thresholds result in new timing rules for maximizing long-term potentiation. Neuropharmacology 2013;64:27–36.
Cao G, Harris KM. Augmenting saturated LTP by broadly spaced episodes of theta-burst stimulation in hippocampal area CA1 of adult rats and mice. J Neurophysiol. 2014;112:1916–24.
Goldsworthy MR, Pitcher JB, Ridding MC. The application of spaced theta burst protocols induces long-lasting neuroplastic changes in the human motor cortex. Eur J Neurosci. 2012;35:125–34.
Müller-Dahlhaus F, Ziemann U. Metaplasticity in human cortex. Neuroscientist 2015;21:185–202.
Thomson AC, de Graaf TA, Kenis G, Rutten BPF, Schuhmann T, Sack AT. No additive meta-plasticity effects of accelerated iTBS with short inter-session intervals. Brain Stimul. 2019;12:1301–3.
Baeken C. Accelerated rTMS: A potential treatment to alleviate refractory depression. Front Psychol. 2018;9:2017.
Cole EJ, Stimpson KH, Bentzley BS, Gulser M, Cherian K, Tischler C, et al. Stanford accelerated intelligent neuromodulation therapy for treatment-resistant depression. Am J Psychiatry. 2020;177:716–26.
McGirr A, Van den Eynde F, Tovar-Perdomo S, Fleck MPA, Berlim MT. Effectiveness and acceptability of accelerated repetitive transcranial magnetic stimulation (rTMS) for treatment-resistant major depressive disorder: An open label trial. J Affect Disord. 2015;173:216–20.
Huang Y-Z, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron 2005;45:201–6.
Corp DT, Bereznicki HGK, Clark GM, Youssef GJ, Fried PJ, Jannati A, et al. Large-scale analysis of interindividual variability in theta-burst stimulation data: Results from the ‘Big TMS Data Collaboration’. Brain Stimul. 2020;13:1476–88.
Schilberg L, Schuhmann T, Sack AT. Interindividual variability and intraindividual reliability of intermittent theta burst stimulation-induced neuroplasticity mechanisms in the healthy brain. J Cogn Neurosci. 2017;29:1022–32.
Goldsworthy MR, Hordacre B, Rothwell JC, Ridding MC. Effects of rTMS on the brain: is there value in variability? Cortex 2021;139:43–59.
Nettekoven C, Volz LJ, Kutscha M, Pool E-M, Rehme AK, Eickhoff SB, et al. Dose-dependent effects of Theta Burst rTMS on cortical excitability and resting-state connectivity of the human motor system. J Neurosci. 2014;34:6849–59.
Tse NY, Goldsworthy MR, Ridding MC, Coxon JP, Fitzgerald PB, Fornito A, et al. The effect of stimulation interval on plasticity following repeated blocks of intermittent theta burst stimulation. Sci Rep. 2018;8:8526.
Yu F, Tang X, Hu R, Liang S, Wang W, Tian S, et al. The after-effect of accelerated intermittent theta burst stimulation at different session intervals. Front Neurosci. 2020;14:576.
Huang Y-Z, Chen R-S, Rothwell JC, Wen H-Y. The after-effect of human theta burst stimulation is NMDA receptor dependent. Clin Neurophysiol. 2007;118:1028–32.
Teo JTH, Swayne OB, Rothwell JC. Further evidence for NMDA-dependence of the after-effects of human theta burst stimulation. Clin Neurophysiol. 2007;118:1649–51.
Cole J, Sohn MN, Harris AD, Bray SL, Patten SB, McGirr A. Efficacy of adjunctive D-Cycloserine to intermittent theta-burst stimulation for major depressive disorder: a randomized clinical trial. JAMA Psychiatry. 2022. https://doi.org/10.1001/jamapsychiatry.2022.3255.
Lakens D. Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front Psychol. 2013;4:1–12.
Forsyth JK, Bachman P, Mathalon DH, Roach BJ, Asarnow RF. Augmenting NMDA receptor signaling boosts experience-dependent neuroplasticity in the adult human brain. Proc Natl Acad Sci USA. 2015;112:15331–6.
Nitsche MA, Jaussi W, Liebetanz D, Lang N, Tergau F, Paulus W. Consolidation of human motor cortical neuroplasticity by D-Cycloserine. Neuropsychopharmacol. 2004;29:1573–8.
Cole J, Selby B, Ismail Z, McGirr A. D-cycloserine normalizes long-term motor plasticity after transcranial magnetic intermittent theta-burst stimulation in major depressive disorder. Clin Neurophysiol. 2021;132:1770–6.
Selby B, MacMaster FP, Kirton A, McGirr A. d-cycloserine blunts motor cortex facilitation after intermittent theta burst transcranial magnetic stimulation: A double-blind randomized placebo-controlled crossover study. Brain Stimul. 2019;12:1063–5.
Groppa S, Oliviero A, Eisen A, Quartarone A, Cohen LG, Mall V, et al. A practical guide to diagnostic transcranial magnetic stimulation: Report of an IFCN committee. Clin Neurophysiol. 2012;123:858–82.
Delvendahl I, Gattinger N, Berger T, Gleich B, Siebner HR, Mall V. The role of pulse shape in motor cortex transcranial magnetic stimulation using full-sine stimuli. PLoS ONE. 2014;9:e115247.
Rossini PM, Burke D, Chen R, Cohen LG, Daskalakis Z, Di Iorio R, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clin Neurophysiol. 2015;126:1071–107.
Lefaucheur JP, Drouot X, Ménard-Lefaucheur I, Keravel Y, Nguyen JP. Motor cortex rTMS restores defective intracortical inhibition in chronic neuropathic pain. Neurology 2006;67:1568–74.
Koller M. robustlmm: An R package for robust estimation of linear mixed-effects models. J Stat Softw. 2016;75:1–24.
Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B (Methodol). 1995;57:289–300.
López-Alonso V, Cheeran B, Río-Rodríguez D, Fernández-Del-Olmo M. Inter-individual variability in response to non-invasive brain stimulation paradigms. Brain Stimul. 2014;7:372–80.
Suppa A, Huang Y-Z, Funke K, Ridding MC, Cheeran B, Di Lazzaro V, et al. Ten years of Theta burst stimulation in humans: Established knowledge, unknowns and prospects. Brain Stimul. 2016;9:323–35.
Dravid SM, Burger PB, Prakash A, Geballe MT, Yadav R, Le P, et al. Structural determinants of d-Cycloserine efficacy at the NR1/NR2C NMDA receptors. J Neurosci. 2010;30:2741–54.
Sheinin A, Shavit S, Benveniste M. Subunit specificity and mechanism of action of NMDA partial agonist D-cycloserine. Neuropharmacology 2001;41:151–8.
Kweon J, Vigne M, Jones R, George MS, Carpenter LL, Brown JC. A replication study of NMDA receptor agonism sufficiency to enhance 10-Hz rTMS-induced motor cortex plasticity. Brain Stimul: Basic, Transl, Clin Res Neuromodulation. 2022;15:1372–4.
Rouaud E, Billard J-M. D-Cycloserine facilitates synaptic plasticity but impairs glutamatergic neurotransmission in rat hippocampal slices. Br J Pharmacol. 2003;140:1051–6.
Di Lazzaro V, Oliviero A, Profice P, Saturno E, Pilato F, Insola A, et al. Comparison of descending volleys evoked by transcranial magnetic and electric stimulation in conscious humans. Electroencephalogr Clin Neurophysiol/Electromyogr Mot Control 1998;109:397–401.
Hamada M, Murase N, Hasan A, Balaratnam M, Rothwell JC. The role of interneuron networks in driving human motor cortical plasticity. Cereb Cortex. 2013;23:1593–605.
Di Lazzaro V, Oliviero A, Saturno E, Pilato F, Insola A, Mazzone P, et al. The effect on corticospinal volleys of reversing the direction of current induced in the motor cortex by transcranial magnetic stimulation. Exp Brain Res. 2001;138:268–73.
Sommer M, Ciocca M, Chieffo R, Hammond P, Neef A, Paulus W, et al. TMS of primary motor cortex with a biphasic pulse activates two independent sets of excitable neurones. Brain Stimul. 2018;11:558–65.
Di Lazzaro V, Profice P, Ranieri F, Capone F, Dileone M, Oliviero A, et al. I-wave origin and modulation. Brain Stimul. 2012;5:512–25.
Karabanov A, Ziemann U, Hamada M, George MS, Quartarone A, Classen J, et al. Consensus paper: Probing homeostatic plasticity of human cortex with non-invasive transcranial brain stimulation. Brain Stimul. 2015;8:993–1006.
Hordacre B, Goldsworthy MR, Vallence A-M, Darvishi S, Moezzi B, Hamada M, et al. Variability in neural excitability and plasticity induction in the human cortex: A brain stimulation study. Brain Stimul. 2017;10:588–95.
Hinder MR, Goss EL, Fujiyama H, Canty AJ, Garry MI, Rodger J, et al. Inter- and intra-individual variability following intermittent theta burst stimulation: implications for rehabilitation and recovery. Brain Stimul. 2014;7:365–71.
Turrigiano GG. Homeostatic plasticity in neuronal networks: the more things change, the more they stay the same. Trends Neurosci. 1999;22:221–7.
Acknowledgements
The authors would like to acknowledge the technical contribution of the Non-invasive neurostimulation Network (N3) at the University of Calgary.
Funding
This study was funded through the Brain and Behaviour Research Foundation Young 2018 Investigator Award (27548) and the Campus Alberta Innovates Program Chair in Neurostimulation.
Author information
Authors and Affiliations
Contributions
JGW, JC, MNS, and AM made substantial contributions to the study conception, data acquisition and analysis and interpretation of the work. All authors were involved in the drafting and final approval of the manuscript and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
JGW, JC, and MNS have no competing interests. AM has a provisional method of use patent application for the combination of D-Cycloserine with intermittent theta-burst stimulation for depression and obsessive-compulsive disorder.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wrightson, J.G., Cole, J., Sohn, M.N. et al. The effects of D-Cycloserine on corticospinal excitability after repeated spaced intermittent theta-burst transcranial magnetic stimulation: A randomized controlled trial in healthy individuals. Neuropsychopharmacol. 48, 1217–1224 (2023). https://doi.org/10.1038/s41386-023-01575-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-023-01575-7
This article is cited by
-
Targeting metaplasticity mechanisms to promote sustained antidepressant actions
Molecular Psychiatry (2024)