Abstract
Antipsychotics are thought to improve schizophrenia symptoms through the antagonism of dopamine D2 receptors, which are abundant mainly in subcortical regions. By introducing functional gradient, a novel approach to identify hierarchy alterations by capturing the similarity of whole brain fucntional connectivity (FC) profiles between two voxels, the present study aimed to characterize how the subcortical gradient is associated with treatment effects and response in first-episode schizophrenia in vivo. Two independent samples of first-episode schizophrenia (FES) patients with matched healthy controls (HC) were obtained: the discovery dataset included 71 patients (FES0W) and 64 HC at baseline, and patients were re-scanned after either 6 weeks (FES6W, N = 33) or 12 months (FES12M, N = 57) of antipsychotic treatment, of which 19 patients finished both 6-week and 12-month evaluation. The validation dataset included 22 patients and 24 HC at baseline and patients were re-scanned after 6 weeks. Gradient metrics were calculated using BrainSpace Toolbox. Voxel-based gradient values were generated and group-averaged gradient values were further extracted across all voxels (global), three systems (thalamus, limbic and striatum) and their subcortical subfields. The comparisons were conducted separately between FES0W and HC for investigating illness effects, and between FES6W/FES12M and FES0W for treatment effects. Correlational analyses were then conducted between the longitudinal gradient alterations and the improvement of clinical ratings. Before treatment, schizophrenia patients exhibited an expanded range of global gradient scores compared to HC which indicated functional segregation within subcortical systems. The increased gradient in limbic system and decreased gradient in thalamic and striatal system contributed to the baseline abnormalities and led to the disruption of the subcortical functional integration. After treatment, these disruptions were normalized and the longitudinal changes of gradient scores in limbic system were significantly associated with symptom improvement. Similar illness and treatment effects were also observed in the validation dataset. By measuring functional hierarchy of subcortical organization, our findings provide a novel imaging marker that is sensitive to treatment effects and may make a promising indicator of treatment response in schizophrenia.
Similar content being viewed by others
Introduction
Identifying biomarkers indicative of treatment response in patients with schizophrenia has been a sustained area of research over the past two decades. Commonly used antipsychotics are thought to improve symptoms via the blockade of dopamine D2 receptors [1, 2] which are abundant in the striatum [3]. Though the development of neuroimaging acquisition and analysis techniques has led to major progress in characterizing the subcortical changes including striatum in anatomy, function and chemistry before and after antipsychotic treatment, the specific regions and related biological measures have yet to show consistency in relation to treatment response.
Structural studies demonstrated that antipsychotic treatment led to gray matter enlargement in striatum and thalamus in schizophrenia patients that associated with the improvement of positive symptoms [4,5,6]. However, striatal and hippocampal volume loss was also found in first-episode schizophrenia (FES) patients after antipsychotic medication [7]. In functional studies, antipsychotic exposure increased the thalamo-cortical connectivity that was disrupted in patients at baseline [8]. Increased regional intrinsic activity in caudate was also associated with improved positive symptoms after antipsychotic treatment [9], while alterations in dorsal vs. ventral striatal connectivity were linked to the improvements of negative and positive symptoms, respectively [10]. At the neurochemical level, longitudinal proton magnetic resonance spectroscopy studies reported that antipsychotic drugs might also increase N-acetylaspartate levels in the thalamus [11, 12].
While these prior findings established the importance of subcortical regions in relation to antipsychotic treatment effects, the reported correlations between the in vivo imaging measures and symptom improvements have been modest. Many previous studies adopted regional measures or altered functional connectivity (FC) between pairs of regions that might lack robust sensitivity to detect subtle changes or correlates with treatment [4, 5, 13]. Additionally, variability in the predefined categorization of treatment response (i.e., responders vs. non-responder) might also contribute to discrepancies across previous studies [14].
Recently, a novel gradient-based approach has been introduced to define a non-linear decomposition of high-dimensional resting-state FC [15]. Unlike the regional analyses, this method can comprehensively identify subcortical functional hierarchies by representing brain connectivity in a continuous, low-dimensional space [16,17,18]. The concept of gradient focuses on connectomes where voxels with similar connectivity patterns are located close to one another along a given connectivity gradient [19]. It has shown some success in defining the hierarchical organization of cerebral cortex, striatum, and hippocampus [17, 20, 21], and elucidating functional gradient relationships with behavior or cognitive functions. This approach has also been used to investigate the reduced FC differentiation between anterior and posterior insula in schizophrenia, revealing that reduced connectional diversity across the insula might underpin the development of psychotic symptoms of patients [22]. Therefore, examining this synchronous measure of subcortical FC architecture in untreated schizophrenia patients and then in relation to symptom improvement after treatment might providing novel insight of illness- and treatment-related effects on subcortical regions.
With these considerations in mind, in this current study, we recruited two independent samples of FES to investigate the pattern of the principal connectome gradient alterations in the subcortex and their relationships with symptoms before and after antipsychotic treatment. The potential and reliability of gradient in relating to treatment response, measured by symptom improvement with continuous variables, will be examined during the analyses.
Methods
Participants
The study was approved by the Ethics Committee of West China Hospital of Sichuan University. All participants provided written informed consent after the study procedures were fully explained. Two main datasets were used in our study, both including a group of patients with FES who had no history of antipsychotic treatment prior to the initial scan and a demographically matched healthy control (HC) group. Diagnosis of schizophrenia was determined using the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) Patient Edition and was confirmed at follow-up. Duration of illness was determined by the Nottingham Onset Schedule [23] according to information provided by patients, family members, and medical records. Clinical symptoms were evaluated by the Positive and Negative Syndrome Scale (PANSS) [24] and the Global Assessment of Functioning (GAF) [25]. The PANSS subscales were further calculated with the five-factor model [26], which captured the critical symptom dimensions of positive, negative, disorganization, excitement and depression.
The discovery dataset was recruited from the Mental Health Centre of the West China Hospital. More than 200 FES patients at baseline were scanned and used in our previous studies [27, 28]. However, only 71 were followed and re-scanned after either 6 weeks (FES6W, N = 33) or 12 months (FES12M, N = 57) of antipsychotic treatment thus were included in this study (Table 1), and 19 of them completed both 6-week and 12-month follow-up scans (see Supplementary Materials and Table S1). The validation dataset was recruited from the Fourth People’s Hospital of Chengdu, 96 FES patients were scanned at baseline but only 22 patients finished the 6-week follow-up, and those patients with longitudinal data were enrolled (Table 2). None of the patients received any other type of treatment other than antipsychotics including aripiprazole, clozapine, olanzapine, quetiapine, risperidone, haloperidol and sulpiride during following-up, and the chlorpromazine-equivalent (CPZ) dosage of each antipsychotic was calculated for all patients [29].
The HC participants were recruited via poster advertisements from the same areas where patients resided, with similar socioeconomic backgrounds to the patients. They were screened using the Structured Clinical Interview for DSM-IV Non-Patient Edition to confirm the lifetime absence of Axis I disorder. Sixty-four matched HCs were included in the discovery dataset and 24 in the validation dataset, who had no known history of psychiatric illness in first-degree relatives. The following exclusion criteria applied to all participants: history of neurological disorders, alcohol/drug abuse or dependence, or any major medical illness.
Data acquisition
The MRI scanning of participants in the discovery dataset was conducted on a GE Signa EXCITE 3.0T scanner (GE Healthcare, Milwaukee, Wisconsin) with an 8-channel phase array head coil, while the validation dataset was acquired on a Trio 3.0T MR scanner (Siemens Medical Systems, Germany) with a 32-channel head coil. Resting-state functional MRI data and high-resolution T1-weighted images (T1WI) were obtained for all participants. Detailed scanning protocols for each dataset were presented in the Supplementary Materials. The obtained brain imaging was inspected by two experienced neuroradiologists to make sure no gross brain abnormalities in any participant.
Data preprocessing
Functional data of the discovery dataset was preprocessed using DPARSFA (Version 4.3, http://rfmri.org/DPARSF) [30] based on the Statistical Parametric Mapping software (SPM 12, http://www.fil.ion.ucl.ac.uk/spm/software/spm12/). Preprocessing included the following steps: removal of first five dummy volumes, slice time and head motion correction (Friston 24) [31], realignment, segmentation, normalization to the Montreal Neurologic Institute space, bandpass filter (0.01–0.10 Hz) and spatial smoothing (full width at half maximum = 4 mm). Notably, the scanning protocols of validation dataset followed those suggested by Human Connectome Project, data preprocessing was thus conducted with suggested pipelines. Details of data processing for each dataset were in the Supplementary Materials.
Considering the potential head motion effects, we obtained the quantified framewise displacements (FD) for all participants and related to gradient scores as noted below, but no effects were observed. Details were added in the Supplementary Materials.
Functional connectome and subcortical gradient mapping
After data preprocessing, the individual subcortical-cortical FC matrix was constructed using Pearson’s correlation between the time courses of each voxel. Considering the different parameters of discovery and validation dataset, especially the preprocessed images were normalized to the different voxel size (3 × 3 × 3 mm3 for discovery dataset and 2 × 2 × 2 mm3 for validation dataset), both subcortical and cortical mask were resliced separately in 3 mm and 2 mm. Notably, though the voxel size and voxel-level FC matrix were different between the two datasets, the region-wise gradient metrics could be calculated for each dataset respectively and it is reasonable to conduct the replication analysis with the same atlas. In addition, if the validation dataset with different scanning and preprocessing procedures could reproduce the findings of discovery dataset, it may further indicate the reliability of the gradient measures.
The atlas of human subcortex was based on principal functional gradient [32], which recapitulated well-known anatomical subcortical nuclei (hippocampus, amygdala, thalamus, nucleus accumbens, globus pallidus, caudate and putamen). We used this atlas to define subcortical spatial extent (resulting in a total of 2364 voxels with 3 mm cubic resolution for discovery dataset and 7984 voxels with 2 mm cubic resolution for validation dataset), and the Schaefer’s Parcellation [33] to define cortical gray matter. The correlation matrix for each participant was then constructed with these templates to characterize the subcortical-cortical functional connectome.
Gradient mapping was generated in the BrainSpace Toolbox (http://github.com/MICA-MNI/BrainSpace) [15], where principal gradients that accounted for primary variability in spatial distribution of subcortical voxel-wise FC were calculated. Six main parameters were entered to the toolbox, including (1) sparsity parameter: 90, (2) kernel: normalized angle, (3) approach: diffusion embedding, (4) alpha parameter: 0.5, (5) alignment: Procrustes analysis, and (6) number of components: 10. Specifically, only the top 10% of connections per row were used to calculate a cosine matrix which captured the similarity in connectivity profiles between voxels. Then, we computed normalized angle based on the abovementioned subcortical-cortical functional connectome, which scales the angle between each pair of voxels as a function of similarity and the normalized angle matrix was fed into the diffusion map embedding algorithm [34]. Compared to other non-linear manifold learning techniques, this algorithm is relatively insensitive to noise and computationally inexpensive [35]. Notably, it is controlled by a single parameter α, which controls the influence of the density of sampling points on the manifold (α = 0, maximal influence; α = 1, no influence).In line with previous studies [17, 21], we set α = 0.5, a choice that retains the global relations between data points in the embedded space and is suggested relatively robust and insensitive to noise in the covariance matrix. In this embedding space, strongly interconnected brain regions are defined either by many connections or few very strong connections that have many and/or strong connections which are closely located, while regions with little and/or weak inter-connectivity are farther apart [17, 34]. Due to the randomness of the direction of yielding gradients, we used an average connectivity matrix calculated from all patients and controls to produce a group-level gradient component template. The individual gradient was realigned to the template using Procrustes rotation [36].
Extraction of principal gradient scores at global-, system- and subfield-level
The first ten gradient components were generated by the default setting. However, given that the principal gradient is thought to represent the most explained variance, and widely applied in previous studies [17, 19], we primarily focused on the measures within that first gradient component. Second and third gradient components as exploratory analyses were displayed in Supplementary Materials. As for the first gradient component, the spatial patterns of the group-averaged principal gradient maps were created to characterize the subcortical hierarchy organization discrepancy and the global-level gradient scores of each group. The global scores represented the scales of the group spatial distribution with each voxel anchored in an abstract space according to its gradient score.
Then, to locate the dominant subcortical systems resulting from the hierarchy disorganization, the group-averaged global subcortical gradient scores were classified into three main neuroanatomical systems: the limbic system (hippocampus and amygdala), thalamic system (thalamus) and striatal system (nucleus accumbens, globus pallidus, caudate and putamen). Further analyses at the subfield level were conducted to identify the alterations in specific subcortical subfields if the abovementioned systems were found with abnormality. We used the subcortex atlas of Scale II, which segments the human subcortex along anterior-posterior/lateral-medial gradient axis [32], delineating 16 subfield regions bilaterally (anterior/posterior hippocampus (a/pHIP); lateral/medial amygdala (l/mAMY), dorsoposterior/ventroposterior/ventroanterior/dorsoanterior thalamus (THA-DP/VP/VA/DA), nucleus accumbens, shell/core (NAc-shell/core), anterior/posterior globus pallidus (a/pGP), anterior/posterior putamen (a/pPUT), anterior/posterior caudate (a/pCAU).
Statistical analysis
Group comparisons of functional connectome gradients
To characterize the hierarchical organization of subcortex in relation to illness and treatment effects, the global-level gradient distribution of subcortical regions was compared between group-averaged maps by using two-sample Kolmogorov-Smirnov tests to examine the cumulative distribution rather than the mean value of groups, as used in previous studies [37]. We first compared the global scores in FES0W with HC to determine the illness effects in patients at baseline, and further examined the treatment effects after 6-week and 12-month treatment by comparing FES6W and FES12M to FES0W, respectively.
Then, the system-level gradient differences of group-averaged maps, which measure the mean differences of gradient value in each group, were compared by using paired t tests across the voxels in the aforementioned group comparisons. System(s) with significant effects of illness or treatment were further examined at subcortical subfield level also with paired t tests. False discovery rate (FDR) corrections were used to correct for multiple comparisons and the statistical significance threshold was set at p < 0.05 with FDR correction.
Correlations between altered gradients and clinical variables
We conducted Pearson’s correlations between abnormal gradient scores in patients at baseline with clinical variables, including duration of illness, CPZ and symptom severity (PANSS and GAF scores), controlling for age, sex and years of education. To further determine the clinical relevance of altered principal connectome gradient patterns in patients after antipsychotic treatment, we correlated the symptom improvement (PANSS and GAF score changes) with the longitudinal alterations of gradient scores at system- and subfield-level using Pearson’s correlation. The statistical significance threshold was set at p < 0.05 with FDR correction.
Validation analysis
In the validation dataset, we repeated our analyses to confirm the reproducibility of the primary results of illness and 6-week treatment effects respectively. The same correlation analyses were conducted to characterize the baseline and longitudinal gradient relationships.
Results
Principal functional gradient alterations in treatment-naïve schizophrenia
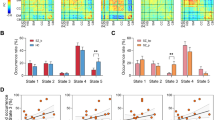
FES0W patients exhibited a wider range of global principal subcortical gradient scores than HC (Kolmogorov-Smirnov test, K-S stat = 2.458, p < 0.001, Fig. 1a), while the gradient map of our HC was consistent with previous characterizations of the spatial distribution of human subcortex [32]. The both highest and lowest gradient scores expanded in patients at baseline compared to HC, which revealed excessive differentiation of subcortical network in the pathophysiology in schizophrenia. The main findings of group-averaged maps in discovery dataset are displayed in Fig. 1b.
a Global histograms showing that gradient range was expanded in schizophrenia at baseline, but normalized after 6-week and 12-month antipsychotic treatment. b The group-averaged maps of principal gradient in discovery dataset, including HC, schizophrenia at baseline as well as after 6-week and 12-month treatment. c System-based histograms showing that the increased limbic and decreased thalamic and striatal gradient contributed to the baseline abnormal global gradient, and the limbic system was normalized the most after both 6-week and 12-month treatment. Blue arrows represent decreased trend of gradient scores while red arrow represents increased trend in system-level in patients at baseline compared to HC. HC healthy controls.
Within neuroanatomical systems, FES0W showed higher gradient scores in the limbic system (t579 = 41.53, p < 0.05, FDR corrected), and lower gradient in the thalamic and striatal systems, relative to HC (t757 = −19.05 and t1025 = −6.11, both p < 0.05 FDR corrected, Fig. 1c), whose systematic discrepancy mainly contributed to the global excessive differentiation. Further characterization of subfields revealed that FES0W had higher gradient scores in bilateral aHIP, pHIP, lAMY and mAMY as well as lower gradient scores in bilateral THA-VP, THA-VA, THA-DA, aCAU, pCAU and left aGP and pPUT compared to HC (all p < 0.05, FDR corrected, Fig. S1). No significant associations were found between abnormal gradient scores in system- or subfield-levels and clinical variables at baseline.
Longitudinal alterations of principal gradients and relation to symptom improvement
At the global level, the wide distribution gap between FES0W and HC was partial narrowed when compared 33 FES6W to HC (K-S stat = 1.512, p = 0.021, Fig. 1a), indicating a normalization process after 6-week antipsychotic treatment and such normalization effects were further enhanced after 12-month treatment (57 FES12M vs. HC, K-S stat = 1.70, p = 0.006, Fig. 1a).
In particular, the increased gradient scores in limbic system at baseline were significantly decreased, while the gradient score reduction in thalamic and striatal system at baseline significantly increased after 6-week treatment (33 FES6W vs. 71 FES0W, t579 = −29.13, t757 = 8.20 and t1025 = 10.56, all p < 0.05, FDR corrected, Fig. 1c). Such longitudinal changes representing a normalizing effect after treatment when compared to HC. However, no association was found between altered gradients after 6-week treatment and longitudinal clinical rating changes.
After 12-month antipsychotic treatment, the gradient scores of limbic system significantly decreased when compared 57 FES12M to 71 FES0W (t579 = 17.99, p < 0.05, FDR corrected, Figs. 1c and 2a), with reduced abnormalities relative to HC. The gradient configuration of thalamic and striatal system in FES12M significantly increased (t757 = 4.41 and t1025 = 9.21, both p < 0.05, FDR corrected, Fig. 1c) and that of the striatal system became comparable to HC (t1025 = 1.43, p = 0.15). The longitudinally decreased principal gradient scores of the limbic system in FES12M were negatively correlated with increase of GAF scores (r = −0.376, p = 0.018, FDR corrected) and positively correlated with reduction of PANSS total scores (r = 0.419, p = 0.006, FDR corrected) and subscales (disorganization scores: r = 0.416, p = 0.030 and excitement scores: r = 0.424, p = 0.030, FDR corrected) (Fig. 2b). Notably, higher scores of the GAF and the lower scores of the PANSS indicated improvement of functioning and symptoms in patients respectively, which showed the opposite longitudinal correlation but presented the same positive relationship between the gradient and symptom improvement.
a Group differences of group-averaged gradient in limbic system in patients between 12-month and baseline. b Longitudinal changes in limbic system were associated with the improvement of GAF, PANSS total scores, disorganization and excitement subscales. c Group differences of group-averaged gradient in subfields of limbic system in patients between 12-month and baseline. ★ The negative correlation coefficient represents consistent improvement in gradient scores and clinical symptoms (both higher scores of the GAF and the lower scores of the PANSS indicated improvement of functioning and symptoms, respectively). HC healthy controls, -rh right hemisphere, -lh left hemisphere, aHIP anterior hippocampus, pHIP posterior hippocampus, lAMY lateral amygdala, mAMY medial amygdala, GAF Global Assessment of Functioning, PANSS Positive and Negative Syndrome Scale.
Since only the limbic system showed relationships between gradient alterations and indicators of treatment response, further analyses were thus conducted in limbic system subfields to identify the specific brain regions or subfield in contributing to treatment response indication. We found that the gradient scores in bilateral a/pHIP and lAMY and right mAMY significantly decreased, with a pattern of change toward being normal (p < 0.05, FDR corrected, Fig. 2c). These changes were also significantly related to symptom improvement as rated by PANSS and GAF scores. Interestingly, the longitudinal decreased gradient scores in right aHIP, l/mAMY and left lAMY were related to PANSS reduction of disorganization and excitement scores in the subscale correlates. More details are in Supplementary Materials and Fig. S2.
Considering that there was some loss of patients during the follow-up in the discovery dataset, we conducted a complementary analysis by repeating the abovementioned analyses with the 19 patients who had evaluations all three time points. The findings were consistent with the main analyses (Figs. S3 and S4) and details were in the Supplementary Materials.
Validation analysis
In the independent validation sample, we found a pattern of principal gradients similar to the primary findings of illness effects and the acute treatment response. At baseline, the FES0W showed higher gradient scores in limbic system (t1999 = 68.48, p < 0.05, FDR corrected) and lower gradient scores in thalamic and striatal system when compared to HC (t2509 = −8.58 and t3473 = −35.70, both p < 0.05, FDR corrected). The gradient scores in FES6W also showed significantly decreased in limbic system (t1999 = −27.71, p < 0.05, FDR corrected), and increased in thalamic and striatal system (t2509 = 4.18 and t3473 = 13.59, both p < 0.05, FDR corrected) compared to FES0W, representing a normalizing process after treatment as compared to the configuration of HC (Fig. S5). The group-averaged maps in validation dataset were displayed in Fig. S6. As also found in discovery dataset, no significant association between such changes with symptom improvement was observed after 6-week treatment.
Discussion
A novel functional connectome gradient algorithm calculating the spatial representation of subcortical functional hierarchy was performed by capturing the similarity of whole brain FC profiles between two voxels. The main finding was that the alterations of gradient scores in subcortical regions in drug-naïve FES patients were normalized after antipsychotic treatment, which were replicated in an independent patient sample. More importantly, the longitudinal changes of the gradients in the limbic system and its subfields were highly associated with improvements in clinical symptoms. These findings extend the importance of subcortex in relation to schizophrenia pathophysiology and antipsychotic treatment in two important aspects: first, the network-based characterization might represent a more sensitive approach to study the effects of illness and antipsychotic treatment effects during the course of illness. Second, the functional subcortical hierarchy disorganization related to the limbic system might represent a robust indicator of treatment response.
Perhaps the most important finding is the normalizing effects of antipsychotic treatment on altered principal functional gradients featured in untreated FES patients. At a global level, the wider gradient range found in drug-naïve patients was indicative of a more differentiated connectivity pattern within subcortical system and may be interpreted as disruption of functional integration during the untreated course of the illness. This might be due to aberrant connectivity patterns that may increase the heterogeneity of subcortical-cortical pathways, which is consistent with prior observations that both excitatory and inhibitory effective connections in subcortex were found in schizophrenia [38]. After treatment, the enlarged physical distance was narrowed and the connectivity dissimilarity was decreased, suggesting that antipsychotics may preferentially impact the subcortex where there is robust express dopamine receptor expression [39, 40].
The normalized gradient of limbic system was found to be highly correlated with treatment response in FES, particularly with the improvement of disorganization and excitement symptom domains. This is consistent with the hypothesis that schizophrenia is in part caused by a high dopaminergic state in the limbic system, where antipsychotic drugs block dopamine receptors to maintain a low dopaminergic tone [41, 42]. Specifically, antipsychotics act on altered neurotransmission and receptor activity and correct intracellular molecular signaling to normalize the functions of neurites, synaptic spines, and synaptogenesis. This is noted to have downstream effects on other neurons [43], an effect related to subcortical interaction [44]. Previous studies have also reported that dopaminergic hyperactivity in subcortical and limbic brain regions could lead to positive symptoms [45, 46], and that decreases in regional cerebral blood flow in limbic circuitry may reflect better treatment response to antipsychotic medications [47]. The functional gradient characterized the limbic system was highly similar connectivity patterns and broader connections with the entire cerebral cortex in patients at baseline, which may indicate a more globally integrated process than single connectome or regional activation studies [9, 48].
We further identified that specific subfields of the hippocampus and amygdala might be primary contributors to the system-level observations. An overdrive of the dopamine system after hippocampal pathology was previously observed in schizophrenia across many neuroimaging and postmortem studies [49,50,51], which could augment dopamine release in extrasynaptic circuits [52, 53]. The hyperactivity in the hippocampus may be weakened by antipsychotic drugs, which could decrease hippocampal excitability, as well as restore normal dopamine neuron population activity [54]. The amygdala is also extensively connected to dopaminergic networks, and affected by dopaminergic signaling [55]. Abnormal amygdala activation in schizophrenia is related to emotion processing dysfunction [56], which could be minimized during antipsychotic treatment [57, 58]. However, in our study, the significant symptom improvement associations of disorganization and excitement domain were detected in relation to decreased gradients of aHIP and l/mAMY but not in pHIP. Along the anterior-posterior hippocampal axis, the aHIP tends to mediate anxiety-related behaviors and the pHIP is implicated in memory and spatial navigation [59, 60], which might account for the observation that only aHIP is linked to the positive-related psychotic symptoms.
The gradient alterations of thalamic and striatal systems were also appeared to normalize after treatment, but the effects were relatively modest. The thalamic system could work with striatal system in dopaminergic modulation [61]. The gradient-based approach [15] characterized the subcortical similarity hierarchy rather than the local function or connectivity, and potentially for this reason, multiple homologous functional subdivisions in striatal and thalamic system may show lower consistency, which further influences the characteristics of gradient in relation to effective treatment response. With respect to striatal system, the ventral “limbic” system is thought to be involved in emotional and reward processing, while a dorsal “associative” system has been defined as a potential link to the dorsal striatum related to associative learning and executive functions [62, 63]. The hierarchy of thalamic system was identified to be along the anterior/posterior axis, with behavioral characterization from lower level perception to higher level cognition [64]. Taken together, no associations of gradient score changes in striatal and thalamic system with the symptom improvement in our study which may be due to our imaging approach that might better characterize the specific similarity in limbic system in schizophrenia and its sensitivity to antipsychotic treatment.
Notably, the normalizing effects of global gradient metrics of subcortical regions were not a pronounced after 6-week treatment as those observed after 1-year treatment. Although the limbic system showed the prominent normalized trend in patients after 6-week treatment, no clinical relevance was found. The gradient corresponded to the intrinsic geometry and indicated that the difference in distances raised discordance among the regional connectivity patterns [15]. There have been previous observations that regional anatomical and functional brain abnormalities were significantly dissociated during the early course of schizophrenia prior to antipsychotic treatment [65]. Considering the inconsistence in the abnormalities of structure and function in FES, the acute treatment effects may not be sufficient to reorganize the synchronization on subcortical gradients along with the improvement of psychiatric symptoms. In addition, the limited statistical power with small sample size of patients with 6-week treatment may be another reason.
Notwithstanding its implications, several limitations of this study should be acknowledged. First, our analysis focused on the first functional gradient as did in most previous studies, since the first component explained the most variance and showed the greatest potential for clinical relevance in current study. The second and third components tended to represent some particular features or effects (Figs. S7 and S8) that require further investigation. Second, during the correlation analysis, we only incorporated symptom improvement and gradient metrics in the model, our results thus were mainly interpreted as a general assessment of treatment response in routine clinical practice. More work is needed to incorporate specific treatment details including medications and dosage for a personalized treatment response prediction in the future. Third, although we included an independent drug-naïve schizophrenia patient group, the sample size was relatively small. Studies with larger samples are required for further verification and subgroup identification. Fourth, the validation dataset only has patients with evaluations at baseline and after 6-week treatment thus just represents a partial validation. Finally, lower spatial resolution of the currently used fMRI data may cause an oversampling issue in the high-resolution surface-based analysis pipeline. Future research should leverage more advanced imaging technique with higher spatial resolution.
In conclusion, the present study provides novel insights into the principal functional gradient of subcortical systems in relation to the effects of illness and antipsychotic treatment in schizophrenia patients. While treatment effects were observed on all subcortical systems, the most significant effect on limbic system and its subfields suggests that limbic system might represent a sensitive indicator of antipsychotic treatment response.
References
Lehman AF, Lieberman JA, Dixon LB, McGlashan TH, Miller AL, Perkins DO, et al. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161:1–56.
Kapur S, Remington G. Dopamine D(2) receptors and their role in atypical antipsychotic action: still necessary and may even be sufficient. Biol Psychiatry. 2001;50:873–83.
McCutcheon RA, Reis Marques T, Howes OD. Schizophrenia—an overview. JAMA Psychiatry. 2020;77:201–10.
Andersen HG, Raghava JM, Svarer C, Wulff S, Johansen LB, Antonsen PK, et al. Striatal volume increase after six weeks of selective dopamine D(2/3) receptor blockade in first-episode, antipsychotic-naïve schizophrenia patients. Front Neurosci. 2020;14:484.
Yue Y, Kong L, Wang J, Li C, Tan L, Su H, et al. Regional abnormality of grey matter in schizophrenia: effect from the illness or treatment? PLoS ONE. 2016;11:e0147204.
Li M, Chen Z, Deng W, He Z, Wang Q, Jiang L, et al. Volume increases in putamen associated with positive symptom reduction in previously drug-naive schizophrenia after 6 weeks antipsychotic treatment. Psychol Med. 2012;42:1475–83.
Ebdrup BH, Skimminge A, Rasmussen H, Aggernaes B, Oranje B, Lublin H, et al. Progressive striatal and hippocampal volume loss in initially antipsychotic-naive, first-episode schizophrenia patients treated with quetiapine: relationship to dose and symptoms. Int J Neuropsychopharmacol. 2011;14:69–82.
Chopra S, Francey SM, O’Donoghue B, Sabaroedin K, Arnatkeviciute A, Cropley V, et al. Functional connectivity in antipsychotic-treated and antipsychotic-naive patients with first-episode psychosis and low risk of self-harm or aggression: a secondary analysis of a randomized clinical trial. JAMA Psychiatry. 2021;78:994–1004.
Lui S, Li T, Deng W, Jiang L, Wu Q, Tang H, et al. Short-term effects of antipsychotic treatment on cerebral function in drug-naive first-episode schizophrenia revealed by “resting state” functional magnetic resonance imaging. Arch Gen Psychiatry. 2010;67:783–92.
Han S, Becker B, Duan X, Cui Q, Xin F, Zong X, et al. Distinct striatum pathways connected to salience network predict symptoms improvement and resilient functioning in schizophrenia following risperidone monotherapy. Schizophr Res. 2020;215:89–96.
Szulc A, Galinska-Skok B, Waszkiewicz N, Bibulowicz D, Konarzewska B, Tarasow E. Proton magnetic resonance spectroscopy changes after antipsychotic treatment. Curr Med Chem. 2013;20:414–27.
Kubota M, Moriguchi S, Takahata K, Nakajima S, Horita N. Treatment effects on neurometabolite levels in schizophrenia: a systematic review and meta-analysis of proton magnetic resonance spectroscopy studies. Schizophr Res. 2020;222:122–32.
Li W, Li K, Guan P, Chen Y, Xiao Y, Lui S, et al. Volume alteration of hippocampal subfields in first-episode antipsychotic-naive schizophrenia patients before and after acute antipsychotic treatment. Neuroimage Clin. 2018;20:169–76.
Howes OD, McCutcheon R, Agid O, de Bartolomeis A, van Beveren NJ, Birnbaum ML, et al. Treatment-resistant schizophrenia: Treatment Response and Resistance in Psychosis (TRRIP) Working Group Consensus Guidelines on Diagnosis and Terminology. Am J Psychiatry. 2017;174:216–29.
Vos de Wael R, Benkarim O, Paquola C, Lariviere S, Royer J, Tavakol S, et al. BrainSpace: a toolbox for the analysis of macroscale gradients in neuroimaging and connectomics datasets. Commun Biol. 2020;3:103.
Burt JB, Demirtaş M, Eckner WJ, Navejar NM, Ji JL, Martin WJ, et al. Hierarchy of transcriptomic specialization across human cortex captured by structural neuroimaging topography. Nat Neurosci. 2018;21:1251–9.
Margulies DS, Ghosh SS, Goulas A, Falkiewicz M, Huntenburg JM, Langs G, et al. Situating the default-mode network along a principal gradient of macroscale cortical organization. Proc Natl Acad Sci USA. 2016;113:12574–9.
Wang XJ. Macroscopic gradients of synaptic excitation and inhibition in the neocortex. Nat Rev Neurosci. 2020;21:169–78.
Huntenburg JM, Bazin PL, Margulies DS. Large-scale gradients in human cortical organization. Trends Cogn Sci. 2018;22:21–31.
Marquand AF, Haak KV, Beckmann CF. Functional corticostriatal connection topographies predict goal directed behaviour in humans. Nat Hum Behav. 2017;1:0146.
Vos de Wael R, Larivière S, Caldairou B, Hong SJ, Margulies DS, Jefferies E, et al. Anatomical and microstructural determinants of hippocampal subfield functional connectome embedding. Proc Natl Acad Sci USA. 2018;115:10154–9.
Tian Y, Zalesky A, Bousman C, Everall I, Pantelis C. Insula functional connectivity in schizophrenia: subregions, gradients, and symptoms. Biol Psychiatry Cogn Neurosci Neuroimaging. 2019;4:399–408.
Singh SP, Cooper JE, Fisher HL, Tarrant CJ, Lloyd T, Banjo J, et al. Determining the chronology and components of psychosis onset: The Nottingham Onset Schedule (NOS). Schizophr Res. 2005;80:117–30.
Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–76.
Aas IH. Guidelines for rating Global Assessment of Functioning (GAF). Ann Gen Psychiatry. 2011;10:2.
Wallwork RS, Fortgang R, Hashimoto R, Weinberger DR, Dickinson D. Searching for a consensus five-factor model of the Positive and Negative Syndrome Scale for schizophrenia. Schizophr Res. 2012;137:246–50.
Cao H, Wei X, Hu N, Zhang W, Xiao Y, Zeng J, et al. Cerebello-thalamo-cortical hyperconnectivity classifies patients and predicts long-term treatment outcome in first-episode schizophrenia. Schizophr Bull. 2022;48:505–13.
Yang B, Zhang W, Lencer R, Tao B, Tang B, Yang J, et al. Grey matter connectome abnormalities and age-related effects in antipsychotic-naive schizophrenia. EBioMedicine 2021;74:103749.
Andreasen NC, Pressler M, Nopoulos P, Miller D, Ho BC. Antipsychotic dose equivalents and dose-years: a standardized method for comparing exposure to different drugs. Biol Psychiatry. 2010;67:255–62.
Yan CG, Wang XD, Zuo XN, Zang YF. DPABI: data processing & analysis for (resting-state) brain imaging. Neuroinformatics. 2016;14:339–51.
Friston KJ, Williams S, Howard R, Frackowiak RS, Turner R. Movement-related effects in fMRI time-series. Magn Reson Med. 1996;35:346–55.
Tian Y, Margulies DS, Breakspear M, Zalesky A. Topographic organization of the human subcortex unveiled with functional connectivity gradients. Nat Neurosci. 2020;23:1421–32.
Schaefer A, Kong R, Gordon EM, Laumann TO, Zuo XN, Holmes AJ, et al. Local-global parcellation of the human cerebral cortex from intrinsic functional connectivity MRI. Cereb Cortex. 2018;28:3095–114.
Coifman RR, Lafon S, Lee AB, Maggioni M, Nadler B, Warner F, et al. Geometric diffusions as a tool for harmonic analysis and structure definition of data: diffusion maps. Proc Natl Acad Sci USA. 2005;102:7426–31.
Tenenbaum JB, de Silva V, Langford JC. A global geometric framework for nonlinear dimensionality reduction. Science. 2000;290:2319–23.
Langs G, Golland P, Ghosh SS. Predicting activation across individuals with resting-state functional connectivity based multi-atlas label fusion. Med Image Comput Comput Assist Inter. 2015;9350:313–20.
Meng Y, Yang S, Chen H, Li J, Xu Q, Zhang Q, et al. Systematically disrupted functional gradient of the cortical connectome in generalized epilepsy: Initial discovery and independent sample replication. Neuroimage. 2021;230:117831.
Sabaroedin K, Razi A, Chopra S, Tran N, Pozaruk A, Chen Z, et al. Frontostriatothalamic effective connectivity and dopaminergic function in the psychosis continuum. Brain. 2022. https://doi.org/10.1093/brain/awac018.
Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III–the final common pathway. Schizophr Bull. 2009;35:549–62.
Chopra S, Fornito A, Francey SM, O’Donoghue B, Cropley V, Nelson B, et al. Differentiating the effect of antipsychotic medication and illness on brain volume reductions in first-episode psychosis: a longitudinal, randomised, triple-blind, placebo-controlled MRI study. Neuropsychopharmacology. 2021;46:1494–501.
Ceraso A, Lin JJ, Schneider-Thoma J, Siafis S, Tardy M, Komossa K, et al. Maintenance treatment with antipsychotic drugs for schizophrenia. Cochrane Database Syst Rev. 2020;8:Cd008016.
Kraguljac NV, McDonald WM, Widge AS, Rodriguez CI, Tohen M, Nemeroff CB. Neuroimaging biomarkers in schizophrenia. Am J Psychiatry. 2021;178:509–21.
Huang XF, Song X. Effects of antipsychotic drugs on neurites relevant to schizophrenia treatment. Med Res Rev. 2019;39:386–403.
Thornton MA, Hughes EG. Neuron-oligodendroglia interactions: activity-dependent regulation of cellular signaling. Neurosci Lett. 2020;727:134916.
Molina V, Sanz J, Sarramea F, Benito C, Palomo T. Prefrontal atrophy in first episodes of schizophrenia associated with limbic metabolic hyperactivity. J Psychiatr Res. 2005;39:117–27.
Jones CA, Watson DJ, Fone KC. Animal models of schizophrenia. Br J Pharm. 2011;164:1162–94.
Lahti AC, Weiler MA, Holcomb HH, Tamminga CA, Cropsey KL. Modulation of limbic circuitry predicts treatment response to antipsychotic medication: a functional imaging study in schizophrenia. Neuropsychopharmacology. 2009;34:2675–90.
Duan X, Hu M, Huang X, Dong X, Zong X, He C, et al. Effects of risperidone monotherapy on the default-mode network in antipsychotic-naive first-episode schizophrenia: posteromedial cortex heterogeneity and relationship with the symptom improvements. Schizophr Res. 2020;218:201–8.
Tamminga CA, Stan AD, Wagner AD. The hippocampal formation in schizophrenia. Am J Psychiatry. 2010;167:1178–93.
Harrison PJ. The neuropathology of schizophrenia. A critical review of the data and their interpretation. Brain. 1999;122:593–624.
Heckers S, Konradi C. Hippocampal neurons in schizophrenia. J Neural Transm. 2002;109:891–905.
Blaha CD, Yang CR, Floresco SB, Barr AM, Phillips AG. Stimulation of the ventral subiculum of the hippocampus evokes glutamate receptor-mediated changes in dopamine efflux in the rat nucleus accumbens. Eur J Neurosci. 1997;9:902–11.
Legault M, Wise RA. Injections of N-methyl-D-aspartate into the ventral hippocampus increase extracellular dopamine in the ventral tegmental area and nucleus accumbens. Synapse. 1999;31:241–9.
Gill KM, Lodge DJ, Cook JM, Aras S, Grace AA. A novel α5GABA(A)R-positive allosteric modulator reverses hyperactivation of the dopamine system in the MAM model of schizophrenia. Neuropsychopharmacology. 2011;36:1903–11.
Phillips AG, Ahn S, Howland JG. Amygdalar control of the mesocorticolimbic dopamine system: parallel pathways to motivated behavior. Neurosci Biobehav Rev. 2003;27:543–54.
Gur RE, Loughead J, Kohler CG, Elliott MA, Lesko K, Ruparel K, et al. Limbic activation associated with misidentification of fearful faces and flat affect in schizophrenia. Arch Gen Psychiatry. 2007;64:1356–66.
Blasi G, Popolizio T, Taurisano P, Caforio G, Romano R, Di Giorgio A, et al. Changes in prefrontal and amygdala activity during olanzapine treatment in schizophrenia. Psychiatry Res. 2009;173:31–8.
Mier D, Schirmbeck F, Stoessel G, Esslinger C, Rausch F, Englisch S, et al. Reduced activity and connectivity of left amygdala in patients with schizophrenia treated with clozapine or olanzapine. Eur Arch Psychiatry Clin Neurosci. 2019;269:931–40.
Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65:7–19.
Strange BA, Witter MP, Lein ES, Moser EI. Functional organization of the hippocampal longitudinal axis. Nat Rev Neurosci. 2014;15:655–69.
McCutcheon RA, Abi-Dargham A, Howes OD. Schizophrenia, dopamine and the striatum: from biology to symptoms. Trends Neurosci. 2019;42:205–20.
Haber SN. Corticostriatal circuitry. Dialogues Clin Neurosci. 2016;18:7–21.
Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–81.
Yang S, Meng Y, Li J, Li B, Fan YS, Chen H, et al. The thalamic functional gradient and its relationship to structural basis and cognitive relevance. Neuroimage. 2020;218:116960.
Gong Q, Lui S, Sweeney JA. A selective review of cerebral abnormalities in patients with first-episode schizophrenia before and after treatment. Am J Psychiatry. 2016;173:232–43.
Funding
This study was supported by the National Key R&D Program of China (Project Nos. 2022YFC2009901 [to SL] and 2022YFC2009900 [to SL]), the National Natural Science Foundation of China (Project Nos. 82120108014 [to SL], 82071908 [to SL], 82101998 [to WZ], 82102007 [to LY], 81761128023 [to QG], 81621003 [to QG] and 81901828 [to ZY]), Chinese Academy of Medical Sciences (Project No. 2021-I2M-C&T-A-022 [to SL]), Sichuan Science and Technology Program (Project No. 2021JDTD0002 [to SL] and 2021YFS0077 [to LY]), 1.3.5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (Project Nos. ZYYC08001 [to SL] and ZYJC18020 [to SL]), and Post-Doctor Research Project, West China Hospital, Sichuan University (Grant No. 2020HXBH005 [to WZ]). SL acknowledges the support from Humboldt Foundation Friedrich Wilhelm Bessel Research Award and Chang Jiang Scholars (Program No. T2019069).
Author information
Authors and Affiliations
Contributions
Conception: CY, WZ, SL and ZY; Methodological development and statistical analysis: CY, JL and ZY; Data collection, acquisition and interpretation: WZ, LY, SL and QG; Manuscript draft: CY, WZ, JRB and RL; Critical revisions of the manuscript and final approval of this version to be published: all authors.
Corresponding authors
Ethics declarations
Competing interests
WZ consulted to VeraSci. JRB has served as a consultant to OptumRx. The remaining authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, C., Zhang, W., Liu, J. et al. Disrupted subcortical functional connectome gradient in drug-naïve first-episode schizophrenia and the normalization effects after antipsychotic treatment. Neuropsychopharmacol. 48, 789–796 (2023). https://doi.org/10.1038/s41386-022-01512-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-022-01512-0