Abstract
Although group 2 Innate Lymphoid Cells (ILC2s) play important roles in driving the pathogenesis of allergic airway inflammation, the molecular mechanisms regulating ILC2 responses remain to be fully elucidated. Adenosine signaling is emerging as an important factor to limit excessive inflammation and tissue damage, its role in ILC2-driven airway inflammation remains to be understood. Here we identify adenosine as a negative regulator of ILC2s and allergic airway inflammation. Elevation of adenosine was observed in lungs after protease papain challenge. Adenosine receptor A2A was abundantly expressed in lung ILC2s. The adenosine analog NECA significantly suppress ILC2s responses and relieved airway inflammation induced by IL-33 or papain. Conversely, blockage of adenosine synthesis by CD73 inhibitor APCP or deficiency of A2A aggravated murine airway inflammation. Adoptive transfer of ILC2s into immunodeficiency NCG mice demonstrated that the regulation of ILC2 by adenosine was cell intrinsic. Mechanistic studies showed that the effects of adenosine on ILC2s were associated with changes in transcriptional profiling, and the elevation of intracellular cAMP and resulted NF-κB downregulation. These observations indicate that adenosine-A2A signaling is a negative regulator of ILC2s, which confers protection against airway inflammation and represents a novel therapeutic target for controlling asthma.
Similar content being viewed by others
Introduction
Asthma is a common recurrent chronic inflammatory disease of the respiratory tract, that manifests as respiratory restrictive symptoms, such as airway hyperresponsiveness and excessive mucus secretion1. As a consequence of changes in lifestyle and increased exposure to atmospheric and vehicular pollution, the worldwide prevalence of allergic disease has risen notably in recent years, and it is estimated that there are now more than 300 million asthmatics worldwide2.
Excessive type 2 immunity is the major characteristic of asthma3,4, in which type 2 cytokines, such as IL-4, IL-5 and IL-13, induce the infiltration of eosinophils in the respiratory tract, thereby promoting the mucus production by airway epithelial cells and airway hyperresponsiveness. Group 2 innate lymphoid cells (ILC2s) play crucial role in driving innate immunity during allergic airway inflammation5. In contrast to type 2 helper T lymphocytes (Th2), ILC2s do not express antigen-specific receptors and instead mount rapid responses to pathogens or allergen exposure6. Upon exposure to allergens, lung epithelial cells secrete alarmin cytokines, such as IL-33, IL-25 and thymic stromal lymphopoietin (TSLP), which bind to corresponding receptors on the surface of ILC2s, thereby promoting rapid expansion and activation of ILC2s resident in lungs7. Moreover, ILC2s facilitate the activation of Th2 cells during the latter stages of airway inflammation8. Accordingly, ILC2s not only initiate innate immune responses during allergic airway inflammation but also sustain and strengthen adaptive immunity in asthma.
The mechanisms underlying ILC2 regulation have been extensively studied, and it has been established that certain hormones9,10, neurotransmitters and neuropeptides11,12,13,14,15, lipid mediators16,17, nutrients and their metabolites18,19, can act on ILC2s via the corresponding receptors expressed on these cells. At present, however, the detailed mechanisms dictating ILC2s responses have yet to be fully elucidated.
Adenosine is an endogenous purine nucleoside that serves as an important metabolite during energy production, and its concentrations tend to be maintained at relatively low levels under physiological conditions20. Conversely, upon pathological stimulation, such as exposure to hypoxic conditions or tissue inflammation, extracellular adenosine 5ʹ triphosphate (ATP) is released from stressed and injured cells and converted to AMP via the enzyme CD39, and is further dephosphorylated by CD73. The released abundant adenosine modulates the function of a variety of cell types via binding to its receptors and therefore contributes to tissue homeostasis21. Adenosine displays a broad immunoregulatory effects. A range of immune cell types express one or more sub-type adenosine receptors, which are further upregulated during the immune responses22. For example, adenosine can bind to receptors such as A2A and A2B on the surface of lymphocytes and myeloid cells, and further promote immunosuppressive effects via intracellular PKA and NF-κB pathways21.
Although the findings of numerous studies have suggested the potential role of adenosine and its receptors in asthma or other airway inflammatory diseases23, the mechanisms of which remain to be fully elucidated. In asthma patients, the levels of adenosine in bronchoalveolar lavage fluid were considerably higher than those in healthy individuals24. Furthermore, it has been reported that, under the condition of acute pulmonary inflammation, ATP and ADP are released into the extracellular space from different cell types, which are metabolized to produce adenosine and display anti-inflammatory and tissue protective effects25.
Although ILC2s play a pivotal role in airway inflammation and the fact that airway inflammation coincides with an accumulation of adenosine25, there is yet no conclusive evidence to indicate a link between elevated adenosine and ILC2 function. The findings of a recent study have, however, hinted at a possible association, showing that treatment of total bone marrow cells with adenosine receptor agonizts or antagonists in vitro can affect the cytokine production of bone marrow ILC226. This study was, nonetheless, relatively limited in scope and the observations would not sufficiently explain an effect of adenosine on ILC2s under asthmatic condition.
In the present study, it was found that adenosine accumulates during ILC2-mediated airway inflammation. Adenosine suppresses ILC2 responses primarily via binding to A2A receptor on ILC2s. Blocking the synthesis of adenosine during papain-induced airway inflammation exacerbates the severity of allergic airway inflammation, as a consequence of enhanced ILC2 responses. Further investigation reveals that the accumulation of cyclic adenosine monophosphate (cAMP) and inhibition of the NF-κB pathway mediate the effects of adenosine – A2A axis on ILC2. Our findings shed new light on the mechanisms of ILC2 regulation during allergic airway inflammation.
Results
Adenosine accumulated upon papain challenge and ILC2s highly express A2A
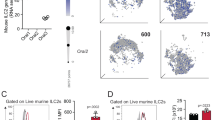
To evaluate the potential role of adenosine in allergic airway inflammation, we measured the levels of adenosine in lungs and bronchoalveolar lavage fluid (BALF) of mice under papain-induced airway inflammation model. Results showed that the amounts of adenosine were dynamically elevated in both lungs and BALF during papain challenge, it gradually increased as the inflammation progresses and decreased as the challenge abort (Fig. 1a, b). Consistent results were observed in the levels of ATP (Supplementary Fig. S1a, b), indicating a heightened metabolism of ATP into adenosine under inflammatory condition. However, no noticeable differences were observed in the transcripts of enzymes responsible for adenosine synthesis between papain-treated and naive control mice, including Entpd1, Nt5e, Enpp1, Cd38, Alpl and Ada, the expression of type 2 cytokines was used as positive control (Supplementary Fig. S1c). Collectively, these results indicate that the local production of adenosine was elevated in response to allergic airway inflammation.
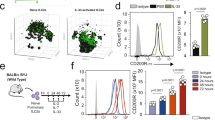
a, b WT mice were treated with papain (20 μg/mouse/day) or PBS intranasally (i.n) for 5 consecutive days, the amount of adenosine in lung tissue or BALF were analyzed at different timepoint (n = 6). c The mRNA expression of adenosine receptors on the indicated mouse ILC subsets and other immune cells, including NK(CD3+NK1.1+NKP46+), ILC1(CD45+Lin−CD127+NK1.1+NKP46+), ILC2(CD45+Lin−CD90.2+CD25+ST2+), ILC3(CD45+Lin−CD127+CCR6+) as well as CD4+T(CD45+CD3+CD4+), CD8+T(CD45+CD3+CD8+) and B lymphocytes (CD45+B220+) determined by RT-PCR. d RT-PCR determined mRNA level of different adenosine receptors expressed on lung ILC2 (CD45+Lin−CD90.2+ CD25+ST2+). e Flow cytometry purified ILC2s (Gated on live CD45+Lin− CD90.2+CD25+ST2+) from lung were cultured in the present of IL-2(20 ng/ml), IL-7(20 ng/ml) with or without IL-33(100 ng/ml) for 3 days, the mRNA expression of Il5, Il13 and adenosine receptors were analyzed by RT-PCR. f Immunofluorescence images of lung tissue from IL-5-Red5 reporter mice analyzed after papain challenge intranasally for 5 consecutive days, stained for DAPI (blue), A2A receptor (green), and IL-5 (red). Red arrows show the A2A+ ILC2, white arrow show the A2A+ non-ILC2 (bars, 20 µm). g Immunofluorescence images of A2B receptor expression on lung tissue, stained for DAPI (blue), A2B (green) and IL-5 (red). Red arrows show the A2B+ ILC2, white arrows show the A2B+ non-ILC2, blue arrows show the A2B− ILC2 (bars, 20 µm). Data are representative of two independent experiments. Error bars show mean ± SEM; *P < 0.05; **P < 0.01; ***P < 0.001 by unpaired Student’s t test.
Considering that ILC2s (Supplementary Fig. S2a) play a primary role in papain-induced airway inflammation, we next investigated the effect of adenosine on ILC2s. Evaluation of distinct adenosine receptors based on RT-PCR analyses revealed that the expression of Adora2a and Adora2b, which encode the A2A and A2B receptors respectively, was highly expressed in ILC2s when compared with other ILC subsets and lymphocytes (Fig. 1c, d). Comparatively, adenosine receptors A1 and A3 show low levels of expression in most types of immune cell (Fig. 1c). Of the A2A and A2B receptors, the former was found to be the most abundantly expressed (Fig. 1d). IL-33 stimulation further upregulated the expression of Adora2a, Adora2b, and effector cytokines produced by ILC2 (Fig. 1e). To further confirmation, IL-5-Red5 reporter mice were used for immunofluorescence staining of adenosine receptors on lung ILC2s. Consistent with previous reports27,28, more than 90% of ILC2s were marked by Red5 in IL-5-Red5 reporter mice and ILC2s constituted nearly 90% of IL-5-Red5+ cells (Supplementary Fig. S3). Results showed that the expression of A2A on lung ILC2s was dramatically increased upon papain challenge (Fig. 1f), no noticeable differences were observed with the expression of A2B (Fig. 1g). These observations indicate a potential role of adenosine/A2A signaling in ILC2-driven airway inflammation.
Adenosine suppresses the responses of ILC2s to IL-33
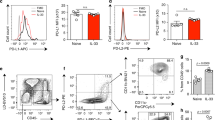
We went on to investigate the potential role of adenosine in ILC2 responses. Given the particular short half-life of adenosine, adenosine analog Adenosine-5’-N-Ethyluronamide (NECA) was used alternatively. Naive mice were challenged with IL-33 intranasally for three consecutive days, and simultaneously treated with either NECA or DMSO (Fig. 2a). In line with expectations, a marked elevation in the number of ILC2 in response to IL-33 challenge was observed, whereas co-treatment with NECA significantly diminished this effect (Fig. 2b). Consistent with the reduction in ILC2 numbers, NECA treatment clearly reduced the proliferation and decreased the effector cytokine production of ILC2s induced by IL-33 (Fig. 2c, d). As a consequence of the impaired ILC2 responses, the number of eosinophils in BALF (Fig. 2e), as well as the concentration of IL-5 and IL-13 cytokines in BALF (Fig. 2f), were consistently reduced after NECA treatment, and histological staining further confirmed that NECA clearly alleviated IL-33 induced airway inflammation (Fig. 2g). These observations provide evidence that adenosine suppresses the responses of lung ILC2s.
a WT mice were challenged with PBS or IL-33 (500 ng/mouse/day) i.n for 3 consecutive days and simultaneously treated with NECA (0.1 mg/kg/day) or DMSO every day intraperitoneally, mice were sacrificed 24 h after last challenge. b Flow cytometric analysis of lung ILC2 (Gated in CD45+). Frequencies of Ki67+ ILC2 (c) and IL-5+IL-13+ ILC2 (d) in lung. e Eosinophils (EOS) (CD45+CD11c−Siglec-F+) levels in BALF were analyzed by flow cytometry. f The amount of IL-5 and IL-13 in BALF was examined by ELISA. g H&E staining of lung tissues (bars, 100 µm). Data are representative of two independent experiments (n = 4 for PBS groups, n = 6 for IL-33 groups). Error bars show mean ± SEM; *P < 0.05; **P < 0.01; ***P < 0.001 by unpaired Student’s t test.
Adenosine alleviates ILC2-driven airway inflammation
The protease papain is widely used to induce ILC2-driven airway inflammation29, we therefore used this model to investigate the effect of adenosine. Mice were intranasally challenged with papain for five consecutive days, during which either NECA or DMSO was administered intraperitoneally (Fig. 3a). Our observations revealed marked reduction in the infiltrated eosinophils and the amounts of type 2 effector cytokines in the BALF of NECA–treated mice as compared with DMSO control under papain condition (Fig. 3b, c). H&E staining of lung tissues confirmed a clear remission of airway inflammation in mice receiving NECA (Fig. 3d). The absolute counts of ILC2s, the proliferation and cytokine production of ILC2 in lungs from NECA-treated mice were consistently decreased as expected (Fig. 3e–g). Our observations thus indicate that NECA alleviates ILC2-mediated airway inflammation.
a WT mice were challenged with PBS or papain i.n for 5 consecutive days and simultaneously treated with NECA or DMSO every day intraperitoneally, mice were sacrificed 24 h after last challenge. b EOS levels in BALF were analyzed by flow cytometry. c The amount of IL-5 and IL-13 in BALF was examined by ELISA. d H&E staining of lung tissues (bars, 100 µm). e Flow cytometric analysis the frequencies and absolute number of ILC2s in lung. f, g Frequencies of Ki67+ ILC2(f) and IL-5+IL-13+ ILC2(g) in lung. Data are representative of two independent experiments (n = 4 for PBS groups, n = 6 for papain groups). Error bars show mean ± SEM; *P < 0.05; **P < 0.01; ***P < 0.001 by unpaired Student’s t test.
In response to airway inflammation, Th2 functions as effector cells during adaptive immunity, and it has been established that there is a mutual regulation between Th2 and ILC230,31. Moreover, adenosine has been reported to regulate Th2 responses32,33. To exclude the potential effect of Th2 cells on adenosine-mediated ILC2s responses, Rag1−/− mice were used in papain model. Similar results were observed, NECA administration reduced eosinophil numbers (Supplementary Fig. S4a) and decreased amounts of effector Th2 cytokines (Supplementary Fig. S4b) in BALF, which was further evidenced by attenuated airway inflammation by H&E staining (Supplementary Fig. S4c). The impaired ILC2 responses after NECA administration was observed as expectedly (Supplementary Fig. S4d–f). These observations indicate that the effect of adenosine on ILC2s is independent of Th2 cells.
Blockage of adenosine synthesis aggravates ILC2-driven inflammation
Based on the aforementioned observations, we speculated that blocking the synthesis of adenosine could enhance the activation of ILC2s and thereby exacerbate the severity of airway inflammation. To this end, we used the CD73-specific inhibitor APCP to block adenosine synthesis in mice. In line with expectations, significant reductions in the amounts of adenosine in lungs and BALF were observed under both PBS and papain treatments (Fig. 4a, b). Importantly, a clear aggravation of airway inflammation induced by papain was observed in APCP-treated mice as compared with control, as indicated by the higher eosinophil infiltration and enhanced levels of IL-5 and IL-13 cytokines in BALF, as well as by the histological scoring of lungs (Fig. 4c–e). Consistent with these observations, ILC2 responses were enhanced by APCP (Fig. 4f–h). Thus, blocking the synthesis of adenosine contributes to an exacerbation of ILC2-driven inflammation.
WT mice were challenged with PBS or papain i.n for 5 consecutive days and simultaneously treated with APCP (20 mg/kg/day) or control vehicle every day intraperitoneally, mice were sacrificed 24 h after last challenge. The amount of adenosine in lung tissue (a) or BALF (b). c EOS plots and absolute number in BALF were analyzed by flow cytometry. d The amount of IL-5 and IL-13 in BALF was examined by ELISA. e H&E staining of lung tissues (bars, 100 µm). f Flow cytometric analysis of lung ILC2. Frequencies of Ki67+ ILC2 (g) and IL-5+IL-13+ ILC2 (h) in lung. Data are representative of two independent experiments (n = 4 for PBS groups, n = 6 for papain groups). Error bars show mean ± SEM; **P < 0.01; ***P < 0.001 by unpaired Student’s t test.
Adenosine suppresses ILC2 responses via A2A receptor
Given the observations that ILC2 expresses A2A and A2B receptors (Fig. 1), we sought to identify which receptor mediates the effect of adenosine on ILC2 responses. To this end, purified lung ILC2s (Supplementary Fig. S2b) were cultured in medium containing IL-2, IL-7 and IL-33 for 3 days in the presence of NECA and/or the A2A antagonist SCH58261, A2B antagonist MRS1754. Consistent with the in vivo observations, administration of NECA suppressed cell proliferation and effector cytokines production of ILC2s in vitro (Fig. 5a–c). Moreover, co-administration of SCH58261 almost completely abrogated the effects of NECA on ILC2 responses, whereas no obvious effects were observed with A2B antagonist MRS1754 (Fig. 5a–c). In line with these observations, A2A agonist CGS21680 suppressed ILC2 responses, whereas A2B agonist BAY 60-6583 displayed no comparable effects (Fig. 5d–f). In a previous work26, different adenosine receptors show different effects on bone marrow (BM) ILC2 that A2B suppress the production of both IL-5 and IL-13, while A2A augment IL-5 production and fail to affect IL-13 release. There, we also analyzed the effects of adenosine on BM ILC2 side by side and showed similar results with this work (Supplementary Fig. S5), which indicated potential tissue-specific differences of adenosine on ILC2. These results indicate that A2A receptor mediates the effects of adenosine on lung ILC2s.
a–c Lung ILC2 were cultured in the presence of IL-2 (20 ng/ml), IL-7 (20 ng/ml) and IL-33 (20 ng/ml) for 3 days and treated with DMSO, NECA (1 μM) + DMSO, NECA (1 μM) + SCH58261(1 μM) or NECA + MRS1754 (2 μM). Flow cytometric analysis of IL-5+IL-13+ (a) and Ki67+ (b) cultured ILC2. c IL-5 and IL-13 amount in supernatant were determined by ELISA. d–f Lung ILC2 were cultured in the presence of IL-2, IL-7 and IL-33 for 3 days and treated with DMSO, NECA (1 μM), CGS 21680 (10 μM) or BAY 60-6583 (10 μM). Flow cytometric analysis of IL-5+IL-13+ (d) and Ki67+ (e) cultured ILC2. f IL-5 and IL-13 amount in supernatant were determined by ELISA. Data are representative of two independent experiments. Error bars show mean ± SEM; *P < 0.05; **P < 0.01; ***P < 0.001 by one-way ANOVA with Bonferroni post-test.
For further confirmation, we went on to examine ILC2 responses in A2A-deficient mice (A2A−/−) using papain model. Compared with WT littermates, A2A−/− mice exhibited aggravated airway inflammatory symptoms in response to papain challenge, as evidenced by a significant elevation of infiltrated eosinophils (Fig. 6a), and higher concentrations of IL-5 and IL-13 (Fig. 6b), in BALF, as well as histological scoring based on H&E staining (Fig. 6c). Consistently, the numbers of ILC2 in lungs, and their proliferation and cytokine production were significantly higher in A2A−/− mice compared with WT control mice (Fig. 6d–f). We further confirmed these observations using an alternative clinically relevant allergen, Alternaria alternata, similar results were observed with papain model (Supplementary Fig. S6). Although ILC2s expressed A2A receptor at steady state, A2A−/− mice displayed comparable levels of ILC2s in distinct tissues at steady state, including lungs, mLN, intestine lamina propria and bone marrow (Supplementary Fig. S7). These results thus indicate that A2A deficiency enhances ILC2 responses and exacerbates airway inflammation.
WT and A2A−/− mice were challenged with PBS or papain i.n for 5 consecutive days and sacrificed 24 h after last challenge. a EOS levels in BALF were analyzed by flow cytometry. b The amount of IL-5 and IL-13 in BALF was examined by ELISA. c H&E staining of lung tissues (bars, 100 µm). d Flow cytometric analysis of lung ILC2. Frequencies of Ki67+ ILC2 (e) and IL-5+IL-13+ ILC2 (f) in lung. Data are representative of two independent experiments (n = 4 for PBS groups, n = 6 for papain). Error bars show mean ± SEM; *P < 0.05; **P < 0.01; ***P < 0.001 by unpaired Student’s t test.
Adoptive transfer of A2A −/− ILC2 into NCG mice aggravates airway inflammation
To evaluate whether the effect of A2A on ILC2 is cell intrinsic, equal numbers of lung ILC2s from A2A−/− and WT mice were transferred into immunodeficient NCG mice, followed by intranasal administration of IL-33 for 3 consecutive days (Fig. 7a). Results revealed that compared with mice receiving WT ILC2s, the recipients of A2A−/− ILC2s were characterized by higher numbers of lung ILC2s (Fig. 7b), higher absolute counts of eosinophils in BALF (Fig. 7c), higher amounts of cytokines in BALF (Fig. 7d), and aggravated lung inflammation (Fig. 7e), thereby providing persuasive evidence that adenosine/A2A signaling suppresses ILC2 responses in a cell-intrinsic manner.
a–e Lung ILC2s (CD45+Lin−CD90.2+ ST2+CD25+) were sorted from IL-33-treated WT or A2A−/− mice and transferred into NCG mice, respectively, followed by IL-33 or PBS i.n. challenge for 3 consecutive days, mice were sacrificed 24 h after last challenge a. b The number of lung ILC2 in recipient NCG mice. c Eosinophils number in BALF of recipient mice. d The amount of IL-5 and IL-13 in BALF. e H&E staining of lung tissues (bars, 100 µm). Data are representative of two independent experiments (n = 4 for PBS groups, n = 6 for IL-33 groups). Error bars show mean ± SEM; *P < 0.05; **P < 0.01; ***P < 0.001 by unpaired Student’s t test.
Higher intracellular cAMP and downregulation of NF-κB signaling explains the effects of adenosine on ILC2s
It has previously been demonstrated that upon binding with the corresponding receptors, adenosine elicits intracellular cAMP production and activates downstream signaling21. Accordingly, to investigate the mechanisms whereby adenosine mediates its effects on ILC2s, NECA- or DMSO-treated ILC2 were subjected to transcriptional analysis by RNA-Seq. Significant upregulation of several genes known to be positively regulated by cAMP was observed in NECA-treated ILC2s as compared with DMSO group (Fig. 8a). Furthermore, ILC2s displayed higher level of cAMP upon NECA treatment, in both the supernatants and lysates (Fig. 8b, c). In addition, administration of the adenylate cyclase inhibitor SQ22536, which blocks cAMP synthesis, was found to significantly abrogate the effects of NECA on ILC2 responses, including cell proliferation and cytokine production (Fig. 8d–f). Collectively, these observations thus indicate that intracellular cAMP mediates the effects of adenosine on ILC2s.
a Lung ILC2 were cultured in the presence of IL-2, IL-7 and IL-33 and treated with DMSO or NECA (1 µM) for 24 h, cells were collected for transcriptional profiling analysis by SMART-seq. Heatmap visualization of the distribution of cells from the four different samples and different expressed genes related to cAMP signal. Lung ILC2 were culture in the presence of IL-2, IL-7 and IL-33 with NECA (1 µM) or DMSO treatment for 3 days, the cAMP concentration in supernatants (b) and ILC2 lysate (c) were measured by ELISA. d–f Lung ILC2 were culture in the presence of IL-2, IL-7 and IL-33 with adenylate cyclase inhibitor SQ22536 (10 µM) for 3 days, Flow cytometric analysis of intracellular IL-5+IL-13+ ILC2 (d) and Ki67+ ILC2 (e). The amount of IL-5 and IL-13 in supernatants were measured by ELISA (f). g Heatmap of different expressed genes related to NF-κB signal pathway. h Lung ILC2 were cultured in the presence of IL-2, IL-7 and IL-33 for 6 h and treated with DMSO, NECA (1 µM)+DMSO, NECA + SCH58261 (1 µM) or NECA + NF-κB agonist Betulinic acid (BA,10 µM)/Prostratin(10 µM), Flow cytometric analysis of the p-P65 MFI in ILC2 with indicated treatment. The amount of IL-5 and IL-13 in supernatants were measured by ELISA (i, j). k WT mice were challenged with papain i.n for 5 consecutive days and simultaneously treated with NECA or DMSO every day intraperitoneally, p-P65 MFI in lung ILC2 were analyzed. Data are representative of two independent experiments. Error bars show mean ± SEM; *P < 0.05; **P < 0.01; ***P < 0.001 by unpaired Student’s t test (b, c) or one-way ANOVA with Bonferroni post-test (d–j).
Subsequent analysis of the RNA-seq data revealed that certain positively regulated genes associated with NF-κB signaling, including Rtkn2, Grem1, Il1rap, and Carm134,35,36,37, were downregulated in response to NECA treatment, whereas the expression of some negative regulators in this pathway, including Pycard, Cyp1b1, and Brms138,39,40, was upregulated (Fig. 8g). These results indicate that NF-κB pathway was downstream signaling of cAMP in ILC2s. Furthermore, flow cytometric analysis showed that NECA reduces the level of phosphorylated p65, which was rescued by co-administration of SCH58261 (Fig. 8h). whereas the NF-κB agonizts betulinic acid (BA) and prostratin almost completely abrogated the effects of NECA on ILC2 cells, including the phosphorylation of NF-κB and the production of type 2 cytokines (Fig. 8h–j). For further confirmation in vivo, WT mice were challenged with papain and treated with or without NECA, the level of phosphorylated p65 was evaluated. Consistently, NECA treatment significantly downregulated p-P65 level in ILC2s compared with DMSO control (Fig. 8k). These results indicate that NF-κB pathway mediates the effects of adenosine on ILC2.
Discussion
Adenosine is ubiquitously distributed within the tissues, and extensive studies have established its manifold roles in a variety of systems and diseases. Numerous ligands or antagonists for different adenosine receptors have been developed for the treatment of a number of clinical diseases, such as cerebral and cardiac ischemic diseases, immune and inflammatory disorders and cancer41. Previous studies have shown that adenosine accumulates in the alveolar lavage fluid of asthmatic subjects24, however, its physiological and pathological roles in this regard are largely unknown. In this study, we revealed that the elevation of adenosine during airway inflammation are associated with reduced ILC2s responses and alleviation of airway inflammation. The regulatory effect of adenosine on ILC2s was mediated by A2A receptor.
Adenosine displays immunosuppressive effects via distinct immune cells. In this regard, T cell receptor signaling was reported to upregulate the expression of A2A receptor on T cells42, whereas binding of adenosine to A2A receptors inhibits TCR signal, as well as CD28 costimulatory and IL-2 receptor signals43,44. Similarly, adenosine perturbes B cell receptor and Toll-like receptor 4 (TLR4) signaling, which in turn impairs the activation and survival of B cells45. In innate immunity, TLR signal simultaneously up-regulates the expression of A2A and A2B on macrophages46, whereas adenosine modifies the properties of macrophages, giving rise to tolerogenic phenotype and tumor-promoting “M2-type” behaviors47. Moreover, regulatory T cells are characterized by high expression levels of CD39 and CD73, the key enzymes associated with adenosine production, and exert their immunosuppressive function by producing adenosine48.
The findings of some previous studies have indicated an association between adenosine and ILC2s function. For example, Csoka et al.26 reported that in vitro, deficiencies in both A2A and A2B receptors influences type 2 cytokine IL-5 and IL-13 production by bone marrow cells in response to IL-7 and IL-33 stimulation, with the production of IL-5 being reduced and that of IL-13 increased, thereby implicating adenosine signaling in the cytokine secretion of bone marrow ILC2s. Patel et al.49 revealed that intestinal A2B receptor signaling induces protective anti-helminth type 2 immune responses. A deficiency in A2B receptors was found to be associated with reduction in M2 macrophages and their production of cytokines such as IL-4 and IL-13, thereby suppressing antihelminth immunity. Furthermore, based on a metabolomics analysis, Helou et al.50 established that ILC2 dysfunction attributable to a deficiency in PD-1 was accompanied by perturbed adenosine metabolism. Although the specific outcomes and mechanisms underlying the effects of adenosine signaling on ILC2s remain to be fully elucidated, the findings of the aforementioned studies indicate that the effects of adenosine on type 2 immunity may be context dependent. In the present study, it was found that lung ILC2s express abundant level of adenosine receptors A2A as compared with other types of immune cells. The effects of adenosine on lung ILC2s were investigated.
With respect to asthma, the roles of adenosine and adenosine receptors have been the subject of investigation for decades, although conflicting observations were observed. It has, for example, been long established that inhaled adenosine acts on smooth muscle cells, thereby promoting intense bronchospasms in asthma and chronic obstructive pulmonary disease (COPD) patients51,52. It has also been found that adenosine mediates the pro-inflammatory effects of IL-13 in lung inflammation, IL-13 causes adenosine accumulation, and adenosine deaminase (ADA) treatment significantly alleviates the pro-inflammatory effects of IL-13 by the degradation of adenosine32. However, recent studies paid attention to the anti-inflammatory effects of adenosine in respiratory diseases. Eckle et al.53, for example, reported that during acute lung injury, CD39 and CD73 expressed in lung tissues produced large amounts of adenosine, which showed protective effects and accordingly suggested a therapeutic strategy for inflammatory disease in lungs. In the present study, we observed an accumulation of adenosine in papain-induced airway inflammation model, and that treatment with the adenosine analog NECA can relieve the airway inflammatory. Moreover, blockage of adenosine synthesis or knock out of A2A receptor aggravated ILC2-mediated airway inflammation via cAMP- and NF-κB pathways. Considering that adenosine receptors are widely expressed in distinct types of immune cells, the contribution of other types of immune cells in adenosine-mediated airway inflammation still needs to be further investigated.
In conclusion, this study identify adenosine as an important regulator in ILC2 responses in lung, which provides novel insight into the complicated role of adenosine in airway inflammation.
Methods
Mice
A2A−/− mice (B6/JGpt-Adora2aem1Cd/Gpt) were kindly provided by Dr. Jiangfan Chen from Wenzhou Medical University (Wenzhou, China). NCG mice (NOD-Prkdcem26Cd52Il2rgem26Cd22/Nju) and Rag1−/− mice (B6.129S7-Rag1tm-1Mom/JNju) were purchased from Nanjing Biomedical Research Institute of Nanjing University (Nanjing, China). Red5 (Il5-tdtomato-cre) reporter mice were provided by Dr. Yong Yu from Tongji University (Shanghai, China). All mice were used at the age of 6–8 weeks. All animal experiments were approved by the Institutional Animal Care and Use Committee of Tianjin Medical University.
Murine airway inflammation models
For papain-induced airway inflammation model, mice were challenged with 20 μg papain (in 40 ul PBS/mouse/day, sigma) or PBS for 5 consecutive days intranasally, BALF and lung tissues were analyzed 24 h after last challenge. For IL-33 challenge model, mice were intranasally treated with recombinant mouse IL-33 (500 ng/mouse/day; BioLegend) or PBS for 3 consecutive days, mice were sacrificed 24 h later after the final treatment. For Alternaria alternata–induced airway inflammation, mice were intranasally challenged with Alternaria alternata (100 μg/mouse/day; Greerlabs) for 4 consecutive days and analyzed 24 h later54. For NECA (E2387, sigma) treatment in vivo, mice were intraperitoneally treated NECA (0.1 mg/kg/day, diluted with 200 μl PBS)55 or equal DMSO (diluted with 200 μl PBS, less than 1‰ v/v) daily along with allergen challenge. For APCP (M3763, sigma) treatment in vivo, mice were intraperitoneally treated APCP (20 mg/kg/day, diluted with 200 μl PBS)56 or equal ddH2O daily along with allergen challenge.
Cell isolation
To isolate cells from bronchoalveolar lavage fluids (BALF) and lung tissues, lungs were flushed by 0.5 ml cold PBS twice via a thin tube inserted into a cut made in the trachea as described. Then, lungs were perfused with 20 ml cold PBS. Lung tissues were cut into small pieces and digested with 0.5 mg/ml type I collagenase (Invitrogen, USA) in RPMI-1640 for 1 h at 37 °C with continuous agitation in an incubator. The crude suspensions were further filtered through 70μm cell strainers. Mononuclear cells were obtained by a 40%/80% Percoll (GE Healthcare, Uppsala, Sweden) gradient. Isolation of cells from large and small intestine was performed by digestion in 0.5 mg/ml collagenase and 5 U/ml DNase for 40 min after removal of intestinal epithelial cells. Leukocytes from lamina propria were enriched by a 40%/80% Percoll gradient.
Quantitative real-time PCR (qRT-PCR)
Total mRNA were extracted using TRIzol (Invitrogen) and reverse transcribed with a synthesis kit (Takara). Genes mRNA expression were analyzed by qPCR. The primer sequences were listed in Supplementary Table S1.
ELISA
BALF from lung inflammation mouse models, supernatants from cultured ILC2, lysates of lung tissues and ILC2 cell lysates were collected. ELISA kits were used to measure the amount of IL-5 (Invitrogen) and IL-13 (Invitrogen) in BALF and culture supernatants (without PMA stimulation), adenosine (Biovision) and ATP(Biovision) in lung tissues, cAMP (Biovision) in culture supernatants and ILC2 lysate.
Immunofluorescence staining of lung
WT mice challenged with papain were prepared and transcranial perfused with 40 mL of ice-cold PBS and followed by 40 mL of 4% PFA. Lung tissues were immersed in 20 mL 4% PFA and incubated for 2 days. Transferring the tissues into 30% sucrose in PB solution for Dehydration for 24 h. slicing the lung tissues into 50 μm sections by Leica CM1860. Samples were fixed, blocked, and permeabilized with PBS containing 0.25% Triton X-100 and 1% BSA at room temperature for 30 min, followed with staining. Rabbit anti-adora2a (CST, 94871S), rabbit anti-adora2b (Abcam, ab222901), donkey anti-rabbit Alexa Fluor 647 and DAPI were used. The Primary antibody staining was at 37 °C for 2 h and followed by 4 °C for 24 h. Secondary antibody staining was at room temperature for 1 h. Nuclei were stained with DAPI for 5 min (Thermo Fisher Scientific). Images of representative tissue were captured by a LSM780 and LSM900 confocal microscope and analyzed by Zen software.
H&E staining
Lung lobes were removed and fixed with 4% PFA and embedded in paraffin and sectioned longitudinally at 7 μm. H&E staining was performed for analysis of airway inflammation.
Flow cytometric analysis and sorting
Single cell suspensions were prepared from lung tissues or BALF and incubated with anti-CD16/CD32 before staining with fluorochrome-conjugated antibodies. LIVE/DEAD™ Fixable Aqua Dead Cell Stain Kit, for 405 nm excitation (Thermo Fisher Scientific) was used to exclude dead cells. For ILC2 staining, cells were primarily labeled with biotin-conjugated lineage antibody cocktail including anti-CD3, anti-CD4, anti-CD5, anti-CD8a, anti-Gr1, anti-B220, anti-NK1.1, anti-CD11b, anti-CD11c, anti-Ter119, and anti-TCR-αβ, then stained with streptavidin-fluorochrome antibody and other surface antibody including anti-CD45, anti-CD90.2, anti-CD25 and anti-ST2. For staining of Ki67, cells were fixed and permeabilized (Foxp3/Transcription Factor Staining Buffer Set, Invitrogen), stained with surface markers before incubation with fluorochrome-conjugated antibody against Ki67. For measuring intracellular IL-5 and IL-13 expression, cells were stimulating with 50 ng/ml PMA (Sigma-Aldrich), 1 µg/ml ionomycin (Sigma-Aldrich), and 1 µg/ml brefeldin A in complete RPMI-1640 for 4 h. Cells were labeled with antibodies to surface markers, fixed and permeabilized using an intracellular fixation and permeability kit (Invitrogen), then stained with anti-IL-5 and anti-IL-13.For analysis of p-P65, cells were fixed and permeabilized followed by staining with rabbit anti-p-P65 western blot antibody, then labeled with fluorochrome-conjugated goat anti-rabbit IgG secondary antibody. CytoFLEX S flow cytometer (Beckman Coulter) or MoFlo Astrios EQ (Beckman Coulter) cell sorter was used for samples acquiring or ILC2 sorting, the purity of ILC2 after sorting exceeded 90% and data were analyzed with Flowjo V10.4. Antibodies used are listed in Supplementary Table S2.
ILC2 culture in vitro
For ILC2 function analysis in vitro, 5 × 103 lung ILC2 were cultured in the presence of 100 ng/ml IL-33, 20 ng/ml IL-2 and 20 ng/ml IL-7 for 3 days, the supernatants were collected for IL-5 and IL-13 analyzation by ELISA. For in vitro treatment, agents were used at concentration of 1 μM for NECA26, 1 μM for SCH5826157, 2 μM for MRS175458, 10 μM for CGS2168057, 10 μM for BAY60-658359, 10 μM for SQ2253660, 10 μM for Betulinic acid61 and 10 μM for Prostratin62.
Adoptive transfer of ILC2
Lung ILC2s were sorted from IL-33 intraperitoneally treated WT or A2A−/− mice and intravenously transferred into NCG mice respectively (1.5 × 104 cells/mouse). Mice were then challenged with IL-33 intranasally for 3 consecutive days. Mice were sacrificed and analyzed 24 h later after the last challenge.
Smart-seq2
Five hundred of cultured ILC2s with indicated treatment were lysed in 5 μL 10 × lysis buffer. Libraries were constructed by SMART-Seq2 with two replicates of each treatment63. Briefly, the lysed RNA was denatured at 72 °C for 3 min, followed by a amplified reaction in a mixture of 5 × FS buffer V2, Discover-sc TS oligo V2, and Discover Reverse Transcriptase (42 °C for 90 min, 70 °C for 15 min 4 °C for hold). Mixed the above PCR products with Discover-sc WTA PCR primer, 2 x Discover PCR mix and proceeded a 20 cycles reaction for amplification cDNA. Purified and quantified PCR products were used for sequencing.
Statistics
All data are derived from at least two independent experiments. Statistical analysis was performed with GraphPad Prism 8.0. Results show mean ± SEM and statistical significance was determined by a two tailed unpaired Student’s t test or one-way ANOVA with Bonferroni post-test. P value < 0.05 was considered significant.
Change history
16 February 2022
A Correction to this paper has been published: https://doi.org/10.1038/s41385-022-00489-9
References
Lambrecht, B. N. & Hammad, H. The immunology of asthma. Nat. Immunol. 16, 45–56 (2015).
Papi, A., Brightling, C., Pedersen, S. E. & Reddel, H. K. Asthma. Lancet 391, 783–800 (2018).
Walker, J. A. & McKenzie, A. N. J. TH2 cell development and function. Nat. Rev. Immunol. 18, 121–133 (2018).
Foster, P. S. et al. Modeling TH 2 responses and airway inflammation to understand fundamental mechanisms regulating the pathogenesis of asthma. Immunol. Rev. 278, 20–40 (2017).
Scadding, G. K. & Scadding, G. W. Innate and adaptive immunity: ILC2 and Th2 cells in upper and lower airway allergic diseases. J. Allergy Clin. Immunol. Pr. 9, 1851–1857 (2021).
Kabata, H., Moro, K. & Koyasu, S. The group 2 innate lymphoid cell (ILC2) regulatory network and its underlying mechanisms. Immunol. Rev. 286, 37–52 (2018).
Martinez-Gonzalez, I., Steer, C. A. & Takei, F. Lung ILC2s link innate and adaptive responses in allergic inflammation. Trends Immunol. 36, 189–195 (2015).
Mirchandani, A. S. et al. Type 2 innate lymphoid cells drive CD4+ Th2 cell responses. J. Immunol. 192, 2442–2448 (2014).
Laffont, S. et al. Androgen signaling negatively controls group 2 innate lymphoid cells. J. Exp. Med. 214, 1581–1592 (2017).
Bartemes, K., Chen, C. C., Iijima, K., Drake, L. & Kita, H. IL-33-responsive group 2 innate lymphoid cells are regulated by female sex hormones in the uterus. J. Immunol. 200, 229–236 (2018).
Wallrapp, A. et al. The neuropeptide NMU amplifies ILC2-driven allergic lung inflammation. Nature 549, 351–356 (2017).
Klose, C. S. N. et al. The neuropeptide neuromedin U stimulates innate lymphoid cells and type 2 inflammation. Nature 549, 282–286 (2017).
Cardoso, V. et al. Neuronal regulation of type 2 innate lymphoid cells via neuromedin U. Nature 549, 277–281 (2017).
Moriyama, S. et al. beta2-adrenergic receptor-mediated negative regulation of group 2 innate lymphoid cell responses. Science 359, 1056–1061 (2018).
Wallrapp, A. et al. Calcitonin gene-related peptide negatively regulates alarmin-driven type 2 innate lymphoid cell responses. Immunity 51, 709–723 e706 (2019).
Doherty, T. A. et al. Lung type 2 innate lymphoid cells express cysteinyl leukotriene receptor 1, which regulates TH2 cytokine production. J. Allergy Clin. Immunol. 132, 205–213 (2013).
von Moltke, J. et al. Leukotrienes provide an NFAT-dependent signal that synergizes with IL-33 to activate ILC2s. J. Exp. Med 214, 27–37 (2017).
Spencer, S. P. et al. Adaptation of innate lymphoid cells to a micronutrient deficiency promotes type 2 barrier immunity. Science 343, 432–437 (2014).
Thio, C. L., Chi, P. Y., Lai, A. C. & Chang, Y. J. Regulation of type 2 innate lymphoid cell-dependent airway hyperreactivity by butyrate. J. Allergy Clin. Immunol. 142, 1867–1883 e1812 (2018).
Cekic, C. & Linden, J. Purinergic regulation of the immune system. Nat. Rev. Immunol. 16, 177–192 (2016).
Allard, B., Allard, D., Buisseret, L. & Stagg, J. The adenosine pathway in immuno-oncology. Nat. Rev. Clin. Oncol. 17, 611–629 (2020).
Hasko, G., Linden, J., Cronstein, B. & Pacher, P. Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nat. Rev. Drug Disco. 7, 759–770 (2008).
Brown, R. A., Spina, D. & Page, C. P. Adenosine receptors and asthma. Br. J. Pharm. 153, S446–S456 (2008). Suppl 1.
Driver, A. G., Kukoly, C. A., Ali, S. & Mustafa, S. J. Adenosine in bronchoalveolar lavage fluid in asthma. Am. Rev. Respir. Dis. 148, 91–97 (1993).
Le, T. T. et al. Purinergic signaling in pulmonary inflammation. Front Immunol. 10, 1633 (2019).
Csoka, B. et al. Adenosine receptors differentially regulate type 2 cytokine production by IL-33-activated bone marrow cells, ILC2s, and macrophages. FASEB J. 32, 829–837 (2018).
Dahlgren, M. W. et al. Adventitial stromal cells define group 2 innate lymphoid cell tissue niches. Immunity 50, 707–722 e706 (2019).
Starkey, M. R., McKenzie, A. N., Belz, G. T. & Hansbro, P. M. Pulmonary group 2 innate lymphoid cells: surprises and challenges. Mucosal. Immunol. 12, 299–311 (2019).
Halim, T. Y., Krauss, R. H., Sun, A. C. & Takei, F. Lung natural helper cells are a critical source of Th2 cell-type cytokines in protease allergen-induced airway inflammation. Immunity 36, 451–463 (2012).
Liu, B., Lee, J. B., Chen, C. Y., Hershey, G. K. & Wang, Y. H. Collaborative interactions between type 2 innate lymphoid cells and antigen-specific CD4+ Th2 cells exacerbate murine allergic airway diseases with prominent eosinophilia. J. Immunol. 194, 3583–3593 (2015).
Li, B. W. et al. T cells are necessary for ILC2 activation in house dust mite-induced allergic airway inflammation in mice. Eur. J. Immunol. 46, 1392–1403 (2016).
Blackburn, M. R. et al. Adenosine mediates IL-13-induced inflammation and remodeling in the lung and interacts in an IL-13-adenosine amplification pathway. J. Clin. Invest 112, 332–344 (2003).
Ryzhov, S. et al. Adenosine-activated mast cells induce IgE synthesis by B lymphocytes: an A2B-mediated process involving Th2 cytokines IL-4 and IL-13 with implications for asthma. J. Immunol. 172, 7726–7733 (2004).
Myouzen, K. et al. Functional variants in NFKBIE and RTKN2 involved in activation of the NF-kappaB pathway are associated with rheumatoid arthritis in Japanese. PLoS Genet 8, e1002949 (2012).
Chang, S. H. et al. Excessive mechanical loading promotes osteoarthritis through the gremlin-1-NF-kappaB pathway. Nat. Commun. 10, 1442 (2019).
Chen, R., Li, M., Zhang, Y., Zhou, Q. & Shu, H. B. The E3 ubiquitin ligase MARCH8 negatively regulates IL-1beta-induced NF-kappaB activation by targeting the IL1RAP coreceptor for ubiquitination and degradation. Proc. Natl Acad. Sci. USA 109, 14128–14133 (2012).
Covic, M. et al. Arginine methyltransferase CARM1 is a promoter-specific regulator of NF-kappaB-dependent gene expression. EMBO J. 24, 85–96 (2005).
Chang, M. X., Chen, W. Q. & Nie, P. Structure and expression pattern of teleost caspase recruitment domain (CARD) containing proteins that are potentially involved in NF-kappaB signalling. Dev. Comp. Immunol. 34, 1–13 (2010).
Palenski, T. L., Gurel, Z., Sorenson, C. M., Hankenson, K. D. & Sheibani, N. Cyp1B1 expression promotes angiogenesis by suppressing NF-kappaB activity. Am. J. Physiol. Cell Physiol. 305, C1170–C1184 (2013).
Samant, R. S. et al. Breast cancer metastasis suppressor 1 (BRMS1) inhibits osteopontin transcription by abrogating NF-kappaB activation. Mol. Cancer 6, 6 (2007).
Jacobson, K. A. & Gao, Z. G. Adenosine receptors as therapeutic targets. Nat. Rev. Drug Disco. 5, 247–264 (2006).
Lappas, C. M., Rieger, J. M. & Linden, J. A2A adenosine receptor induction inhibits IFN-gamma production in murine CD4+ T cells. J. Immunol. 174, 1073–1080 (2005).
Bjorgo, E. & Tasken, K. Novel mechanism of signaling by CD28. Immunol. Lett. 129, 1–6 (2010).
Zhang, H. et al. Adenosine acts through A2 receptors to inhibit IL-2-induced tyrosine phosphorylation of STAT5 in T lymphocytes: role of cyclic adenosine 3’,5’-monophosphate and phosphatases. J. Immunol. 173, 932–944 (2004).
Minguet, S. et al. Adenosine and cAMP are potent inhibitors of the NF-kappa B pathway downstream of immunoreceptors. Eur. J. Immunol. 35, 31–41 (2005).
Csoka, B. et al. Adenosine promotes alternative macrophage activation via A2A and A2B receptors. FASEB J. 26, 376–386 (2012).
Ferrante, C. J. et al. The adenosine-dependent angiogenic switch of macrophages to an M2-like phenotype is independent of interleukin-4 receptor alpha (IL-4Ralpha) signaling. Inflammation 36, 921–931 (2013).
Ehrentraut, H. et al. CD73+ regulatory T cells contribute to adenosine-mediated resolution of acute lung injury. FASEB J. 27, 2207–2219 (2013).
Patel, N. et al. A2B adenosine receptor induces protective antihelminth type 2 immune responses. Cell Host Microbe 15, 339–350 (2014).
Helou, D. G. et al. PD-1 pathway regulates ILC2 metabolism and PD-1 agonist treatment ameliorates airway hyperreactivity. Nat. Commun. 11, 3998 (2020).
Cushley, M. J., Tattersfield, A. E. & Holgate, S. T. Inhaled adenosine and guanosine on airway resistance in normal and asthmatic subjects. Br. J. Clin. Pharm. 15, 161–165 (1983).
Oosterhoff, Y., de Jong, J. W., Jansen, M. A., Koeter, G. H. & Postma, D. S. Airway responsiveness to adenosine 5’-monophosphate in chronic obstructive pulmonary disease is determined by smoking. Am. Rev. Respir. Dis. 147, 553–558 (1993).
Eckle, T. et al. Identification of ectonucleotidases CD39 and CD73 in innate protection during acute lung injury. J. Immunol. 178, 8127–8137 (2007).
Xiao, Q. et al. PPARgamma enhances ILC2 function during allergic airway inflammation via transcription regulation of ST2. Mucosal Immunol. 14, 468–478 (2021).
Ryzhov, S. et al. Effect of A2B adenosine receptor gene ablation on adenosine-dependent regulation of proinflammatory cytokines. J. Pharm. Exp. Ther. 324, 694–700 (2008).
Thompson, L. F. et al. Crucial role for ecto-5’-nucleotidase (CD73) in vascular leakage during hypoxia. J. Exp. Med 200, 1395–1405 (2004).
Bonaventura, J. et al. Allosteric interactions between agonists and antagonists within the adenosine A2A receptor-dopamine D2 receptor heterotetramer. Proc. Natl Acad. Sci. USA 112, E3609–E3618 (2015).
He, W., Mazumder, A., Wilder, T. & Cronstein, B. N. Adenosine regulates bone metabolism via A1, A2A, and A2B receptors in bone marrow cells from normal humans and patients with multiple myeloma. FASEB J. 27, 3446–3454 (2013).
Coppi, E. et al. Adenosine A2B receptors inhibit K(+) currents and cell differentiation in cultured oligodendrocyte precursor cells and modulate sphingosine-1-phosphate signaling pathway. Biochem Pharm. 177, 113956 (2020).
Otaka, N. et al. Myonectin is an exercise-induced myokine that protects the heart from ischemia-reperfusion injury. Circ. Res. 123, 1326–1338 (2018).
Brusotti, G. et al. Betulinic acid is a PPARgamma antagonist that improves glucose uptake, promotes osteogenesis and inhibits adipogenesis. Sci. Rep. 7, 5777 (2017).
Abdelnabi, R. et al. Protein kinases C as potential host targets for the inhibition of chikungunya virus replication. Antivir. Res. 139, 79–87 (2017).
Picelli, S. et al. Smart-seq2 for sensitive full-length transcriptome profiling in single cells. Nat. Methods 10, 1096–1098 (2013).
Acknowledgements
This work was supported by grants from National Natural Science Foundation of China (Nos. 81925018, 82130049, 82001748), the Start-up Funding for High-level Talents of Tianjin Medical University, Key Project of Tianjin Natural Science Foundation (20JCZDJC00670).
Author information
Authors and Affiliations
Contributions
Q.X. and X.H. performed experiments, analyzed data, and participated in figure organization and manuscript writing. G.L. and D.Z. participated in the mouse model experiment. L.Z., J.H., H.X., and P.Z. participated in the in vitro cell culture experiments. Q.Y., J.C. participated in manuscript revision and discussion. J.Z., G.J. and Z.Y. conceptualized, supervised, interpreted the experiments and wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: Due to misuse of Suppl. figure 4c.
Supplementary information
Rights and permissions
About this article
Cite this article
Xiao, Q., Han, X., Liu, G. et al. Adenosine restrains ILC2-driven allergic airway inflammation via A2A receptor. Mucosal Immunol 15, 338–350 (2022). https://doi.org/10.1038/s41385-021-00475-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41385-021-00475-7
This article is cited by
-
Ecto-5ʹ-nucleotidase (CD73): an emerging role as prognostic factor in allergic sensitization
Inflammation Research (2024)