Abstract
The gastrointestinal microenvironment, dominated by dietary compounds and the commensal bacteria, is a major driver of intestinal CD4+ T helper (Th) cell differentiation. Dietary compounds can be sensed by nuclear receptors (NRs) that consequently exert pleiotropic effects including immune modulation. Here, we found that under homeostatic conditions the NR Liver X receptor (LXR), a sensor of cholesterol metabolites, regulates RORγt+ CD4 T cells in the intestine draining mesenteric lymph node (MLN). While LXR activation led to a decrease, LXR-deficiency resulted in an increase in MLN Th17 and RORγt+ Tregs. Mechanistically, LXR signaling in CD11c+ myeloid cells was required to control RORγt+ Treg. By contrast, modulation of MLN Th17 was independent of LXR signaling in either immune or epithelial cells. Of note, horizontal transfer of microbiota between LXRα−/− and WT mice was sufficient to only partially increase MLN Th17 in WT mice. Despite LXRα deficiency resulted in an increased abundance of Ruminococcaceae and Lachnospiraceae bacterial families compared to littermate controls, microbiota ablation (including SFB) was not sufficient to dampen LXRα-mediated expansion of MLN Th17. Altogether, our results suggest that LXR modulates RORγt+ Treg and Th17 cells in the MLN through distinct mechanisms.
Similar content being viewed by others
Introduction
The intestinal barrier is continuously challenged by exposure to the external environment. Our immune system has evolved to rapidly adapt to environmental changes by mounting tolerogenic and effector responses on demand. A dynamic equilibrium between these two arms of the immune system is required to maintain homeostasis, and failure in mounting an appropriate regulatory response may lead to uncontrolled inflammatory reactions and chronic immune disorders.
Owing to their ability to react in an antigen-specific manner, CD4+ T helper (Th) cells are central players in ensuring a protective response while limiting collateral damage. Mounting a functional Th cell response in the intestine relies on the anatomical distribution of T cells in immunological inductive and effector sites, where the former is constituted by intestine-draining lymphoid structures such as MLN and the latter being the intraepithelial and lamina propria compartments.1 The inductive sites represent the arena where naïve CD4+ T cells encounter their cognate antigens for the first time and functionally commit to specific effector subsets. Under steady state, retinoic acid-related orphan receptor γt (RORγt)+ Th cells (Th17) and Foxp3 expressing regulatory T cells (Treg) are the most represented Th subsets in the MLN. Despite a certain degree of lineage and functional flexibility, Th17 and Treg cells play complementary roles in maintaining intestinal homeostasis. Although the expression of RORγt and Foxp3 was originally thought to be mutually exclusive, a distinct new population of RORγt+Foxp3+ regulatory T cells (RORγt+ Treg) was described in the mouse intestine,2,3,4 shown to mediate immunosuppression and control Th2 responses at the intestinal barrier.2,3,4
Differentiation to Th17 cells in lymphoid organs is contingent on the presence of TGF-β1, IL-6, and IL-1β cytokines, while their effector function and pathogenicity relies on IL-23. In agreement with their high potential to induce Th17 cell differentiation, intestinal dendritic cells (DCs) are a major source of IL-23, IL-6, and IL-1β.5,6 Of note, mice lacking a specific DC subtype characterized by the expression of CD11b and CD103 have reduced numbers of Th17 cells,6 highlighting the important role of myeloid cells in Th17 cell homeostasis. The intestinal tract represents a hub for the generation of Th17 cells, which in turn have been shown to exert their effector functions both in the intestine and in extra-intestinal sites.7 This phenomenon can be explained by the contribution of environmental signals, mainly diet- and bacterial-derived, in Th17 cell generation. As examples, diets rich in long-chain fatty acids or high in salt content supports Th17 cell differentiation in the gut while germ-free animals have reduced numbers of Th17 cells.8,9,10 Of note, one specific Gram-positive bacterium related to Clostridia, segmented filamentous bacteria (SFB), has proven sufficient to induce Th17 cell development in the intestine.10 By adhering to the intestinal epithelium, SFB drives the production of serum amyloid A, which in turn boosts the expression of IL-6 and IL-23 by antigen presenting cells, ultimately leading to SFB-specific and unspecific Th17 cell generation in the gut.10
At the crossroads between host and environment, NRs are specialized sensors capable of detecting and functionally translating environmental cues by regulating gene transcription. Liver X Receptor (LXR), a member of the NR family, binds to cholesterol metabolites oxysterols and acts as a master regulator of cholesterol homeostasis.11 There are two isoforms of LXR, with LXRβ being ubiquitously expressed and LXRα having tissue specific expression, such as in the liver, adipose tissue, intestine, and macrophages,11 which alone or in combination play critical immunomodulatory roles, such as cytokine production and immunosurveillance against tumors.12,13,14 Both isoforms are expressed and have an antiproliferative effect in human CD4+ T cells,15 implicating a role of LXR in modulating T cells function. Administration of synthetic LXR ligand in vitro and in vivo has been shown to promote Treg cell generation while dampening effector T-cell responses.16 However, other studies have shown no effect in Treg differentiation upon LXR activation in vitro.17 Importantly, activation of both LXR isoforms plays a part in inhibiting Th17 cell generation in a T cell-intrinsic fashion by inducing the expression of Srebp-1, which in turn physically interacts with and inhibits aryl hydrocarbon receptor (Ahr), a known inducer of Th17 cells.18 Consequently, systemic activation of LXR ameliorates symptoms of experimental autoimmune encephalomyelitis (EAE), a Th17-driven mouse model of multiple sclerosis.18 While these findings support the notion of LXR as a regulator of Th17 cell biology, whether LXR modulates intestinal T-cell homeostasis in vivo is yet to be explored. Here, we investigated the relative contribution of different LXR isoforms in controlling intestinal RORγt+ T cells under homeostatic conditions. We found that while LXR activation led to a decrease, LXR deficiency promoted an increase in Th17 and RORγt+ Tregs in the MLN. Mechanistically, we showed that LXR signaling in CD11c+ myeloid cells was sufficient to regulate RORγt+ Treg homeostasis. On the contrary, bone marrow (BM) chimera and conditionally deficient mouse models showed that control of Th17 cells was independent of LXR signaling not only in T cells, but also in immune and intestinal epithelial cells (IECs). Further, while microbiota analysis of LXRα−/− mice showed increased abundance of bacteria belonging to the family Ruminococcaceae and Lachnospiraceae, microbiota ablation (including SFB) was not sufficient to dampen the expansion of Th17 in the MLN of LXRα−/− mice. Overall, our study points towards distinct modes of regulation of RORγt+ T cells by LXR in the MLN.
Results
LXR regulates RORγt+ T cell subsets in the MLN under steady state condition
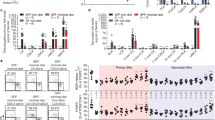
To evaluate the role of LXR in regulating intestinal Th17 cells, C57BL/6J wild-type (WT) mice were fed with a diet containing GW3965, a well-characterized LXR agonist.19 After 10 days, T cell subsets composition in MLN was assessed by flow cytometry. While the proportion of CD3+CD4+ T cells out of total immune cells was unchanged (Fig. 1a), lineage specification into RORγt+Foxp3− (referred as Th17) and RORγt+Foxp3+ (referred as RORγt+ Treg) cell frequencies were reduced by GW3965 administration compared to control diet (Fig. 1b, c). These results suggest that activation of LXR in vivo restrains T cell differentiation to RORγt+ Treg and Th17 cells in the MLN. To further complement these findings, we analyzed the MLN of mice lacking either LXRα (LXRα−/−) or LXRβ (LXRβ−/−) compared to C57BL/6J WT mice obtained from homozygous breeding pairs and same provider (Taconic). In agreement, deficiency of either isoform of LXR resulted in increased proportion of Th17 cells in the MLN (Fig. 1d, e). In contrast, RORγt+ Tregs were increased only in LXRβ-deficient animals compared to both WT and LXRα−/− mice (Fig. 1d, e). To gain further insights into how genetic deficiency in LXRα isoform drives proportional expansion of MLN Th17 cells, we tested whether proliferation (Ki-67 staining) or survival (AnnV staining) of Th17 cells was favored in LXRα−/− mice. Although we did not see differences in Ki-67+ Th17 cells (Fig. 1f), we observed a drop in AnnV+ Th17 cells in LXRα−/− mice (Fig. 1g), suggesting that Th17 cells in LXRα−/− mice have a better survival compared to WT mice. Whether this effect in Th17 survival is specific for the LXRα isoform or lacking LXRβ also results in enhanced Th17 survival remains to be elucidated. Overall, our data showed that LXR regulates RORγt+ T cells in the MLN.
a–c Wild-type (WT) mice were fed either a control or GW3965 containing diet for 10 days followed by analysis of T cells in the MLN. a Frequencies of CD3+ CD4+ T cells out of CD45+ cells in the MLN (n = 10 mice for each group). b, c Representative dot plots (b) and normalized frequencies (c) of RORγt+Foxp3− (Th17) and RORγt+Foxp3+ (RORγt+ Treg) cells out of total CD4+ T cells (n = 10 mice for each group). d, e Representative dot plots (d) and frequencies (e) of Th17 and RORγt+ Treg out of total CD4+ T cells in the MLN of WT (n = 9), LXRα−/− (n = 4) and LXRβ−/− (n = 6) mice purchased from Taconic. f, g Representative dot plots and frequencies of Ki-67+ (f) and AnnexinV+ (g) Th17 cells in the MLN of WT (n = 11) and LXRα−/− (n = 8) mice purchased from Taconic. Data are represented as means ± SD. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 by unpaired Student’s t test or one-way ANOVA with Bonferroni’s post-test when more than two groups.
LXR signaling in DCs is a prerequisite to restrain RORγt + Tregs in the MLN
Next, we investigated whether Th17 expansion in the MLN was dependent on T cell-intrinsic LXR signaling. We first evaluated if LXRα and LXRβ coding transcripts (Nr1h3 and Nr1h2, respectively) were expressed in CD4+ T cells. In line with a previous report on human CD4+ T cells,15 both isoforms were expressed in CD4+ T cells from the MLN, with Nr1h2 (LXRβ) expression being higher compared to Nr1h3 (LXRα) (Supplementary Fig. 1a, b). We then investigated whether LXR signaling modulated T cell differentiation in vitro. Toward this, splenic CD4+ T cells were purified and differentiated in vitro under Th2, Treg, or Th17 polarizing conditions in presence or absence of LXR agonist (GW3965) or antagonist (GSK2033). While LXR inhibition did not affect CD4+ T-cell differentiation towards any of the tested subsets, LXR activation reduced differentiation toward all the tested Th subsets (Supplementary Fig. 2a, b). Further, LXR activation inhibited CD4+ T cell proliferation (Supplementary Fig. 2c, d), in accordance with the previously reported antiproliferative effect of LXR,20 suggesting that defects in Th subset differentiation may be due to a general impairment in proliferation. Thus, in order to assess whether LXR activation in T cells was required to promote Th17 generation in vivo, we used mixed bone marrow (BM) chimera. Given that lack of LXRα resulted in enhanced survival of Th17, we focused on LXRα-deficient mice to further understand its role in MLN Th17 cells. We used WT calibrator BM cells from CD90.1 CD45.2 mice that were mixed in 1:1 ratio with BM cells from LXRα−/− or WT mice (characterized by expression of the congenic markers CD90.2 and CD45.2). Mixed BM cells were intravenously injected into WT CD45.1 lethally irradiated mice generating WT:LXRα−/− and WT:WT mixed BM chimeras, respectively (Fig. 2a). Six-weeks after reconstitution, most MLN-resident immune cells were donor-derived (Supplementary Fig. 3a). Among donor cells, we were able to distinguish RORγt+ CD4+ T cells from the calibrator (CD90.1) and WT or LXRα−/− (CD90.2) BM-derived cells (Supplementary Fig. 3a). Similar to WT:WT chimeras, we found comparable frequencies of RORγt+ CD4+ T cells between WT and LXRα−/− (Fig. 2b, c), indicating that intrinsic deficiency in LXRα does not affect Th17 cell differentiation in the MLN. To further confirm these results, we generated mice lacking LXR in CD4+ T cells (LXRαβΔCD4) by crossing CD4-cre with LXRαβflox/flox mice. As expected, LXRαβΔCD4 did not show any difference in frequencies of Th17 and RORγt+ Treg cells compared to their LXRαβflox/flox littermates (Fig. 2d). Hence, to understand in which cell compartment LXR signaling was required to control MLN RORγt+ T cells, we focused on dendritic cells (DCs), particularly CD103+CD11b+ DCs, due to their ability to activate and promote differentiation of Th17 cells.6 Analysis of published scRNAseq data from the small intestine lamina propria21 revealed that both LXR isoforms were expressed in the cluster corresponding to CD103+CD11b+ cDC2s (defined as Cd11c+Irf4+) whereas only Lxrβ transcripts were found in cell clusters corresponding to cDC1s (defined as Cd11c+Batf3+Irf4neg) and pDCs (defined as Cd11c+Siglech+) (Fig. 2e), suggesting that only cDC2s might signal through LXRα. We thus assessed whether lack of LXR altered DCs homeostasis in the MLN.
a Scheme of the mixed bone marrow chimera experiment: bone marrow (BM) cells from WT or LXRα−/− mice (CD90.2 CD45.2) were mixed in a 1:1 ratio with WT calibrator BM cells (CD90.1 CD45.2) and injected into irradiated WT recipients (CD45.1) for the generation of mixed BM chimeras. Six weeks after transfer, MLN from recipient mice were analyzed by flow cytometry. b, c Representative dot plots (b) and frequencies (c) of RORγt+ cells gated on CD4+ T cells from each donor (data are from one representative experiment out of two independent experiments). d Frequencies of Th17 and RORγt+ Tregs gated on CD4+ T cells in littermate controls (LXRαβflox/flox) and LXRαβΔCD4 (CD4-Cre × LXRαβflox/flox) mice. e Top: relative expression of Cd11c, Siglech, Batf3 and Irf4 (shown in purple) in UMAP analysis of publicly available single-cell RNA sequencing of small intestinal immune cells. Bottom: relative expression of Nr1h3 (LXRα) and Nr1h2 (LXRβ) within the outlined DCs population (cDC1 defined as Cd11c+Batf3+Irf4neg; cDC2 defined as Cd11c+Irf4+ and pDCs defined as Cd11c+Sigleh+). f Frequencies of Th17 and RORγt+ Tregs gated on CD4+ T cells in littermate controls (LXRαβflox/flox) and LXRαβΔCD11c (CD11c-Cre × LXRαβflox/flox) mice. Each dot represents one mouse and data are represented as means ± SEM. ns nonsignificant, **p < 0.01 by unpaired Student’s t test.
Analysis of DCs (defined as CD45+CD11c+CD64negMHC-II+) showed a significant increase in DC frequencies in LXRα−/− mice compared to controls, while total number of DCs were comparable (Supplementary Fig. 4a). Further stratification of DCs using CD103 and CD11b expression resulted in higher numbers of CD103+CD11b+ DCs (cDC2s), but not CD103+ or CD11b+ single positive DCs in the MLN of LXRα−/− mice compared to controls (Supplementary Fig. 4b, c), suggesting that Th17 cell expansion might be controlled by the CD103+CD11b+ DC population. To address this hypothesis, we generated mice lacking LXR in CD11c+ cells (LXRαβΔCD11c), by crossing CD11c-Cre with LXRαβflox/flox mice. Since previous studies reported leakiness of Cre expression using CD11c-Cre mice,22 we first tested Cre specificity using CD11c-cre × Rosa26LSL-EYFP mice. We found that, among lymphocytes, only a minor proportion of B cells and approximately 20% of T cells were EYFP+. In contrast, almost all CD11c+ MHC-II+ cells within the MLN were Cre+ (i.e., expressing EYFP) (Supplementary Fig. 4d). Surprisingly, when we analyzed LXRαβΔCD11c mice and littermate controls, Th17 cell frequencies were comparable, whereas a significant increase in RORγt+ Treg cells was seen in the MLN of mice lacking LXR in CD11c+ cells (Fig. 2f). These results suggest that while LXR signaling in CD11c+ DCs modulate RORγt+ Tregs, LXR signaling neither in T cells nor DCs is required to modulate Th17 cells in the MLN.
LXR signaling is not required in immune or IECs to regulate Th17 cells in MLN
Mucosal Th17 cell responses have been shown to be dependent on both immune and IECs. To further investigate in which compartment LXR activity was necessary to influence the MLN Th17 cells, we generated mice lacking LXR in IECs (LXRαβΔIEC) or immune cells (LXRαβΔVAV) by crossing Villin-Cre and Vav1-iCre with LXRαβflox/flox mice, respectively. Neither LXRαβΔIEC nor LXRαβΔVAV mice showed differences in frequencies of Th17 cells compared to their respective littermate controls (Fig. 3a–d). On the other hand, LXRαβΔVAV mice exhibited a significant increase in frequency of RORγt+ Treg cells compared to littermate controls (Fig. 3c, d), further confirming our findings with LXRαβΔCD11c mice with respect to RORγt+ Treg cells. RORγt+ Treg cells are predominantly found in the colonic lamina propria under steady-state conditions.3 However, LXRαβΔVAV mice showed similar frequencies of RORγt+ Treg cells in the colonic lamina propria compared to the littermate controls, thus suggesting that LXR expression in immune cells controls RORγt+ Treg cells during T-cell priming within the MLN (Supplementary Fig 5). Whether this increase in RORγt+ Treg in the MLN equilibrate in the colonic lamina propria or home to extra-intestinal tissues needs further investigation. Overall, our data suggest that LXR is not required in immune or epithelial cells to modulate Th17 cells in the MLN.
a, b Representative dot plot (a) and frequencies (b) of Th17 (RORγt+Foxp3−), Treg (RORγt−Foxp3+) and RORγt+ Treg (RORγt+Foxp3+) cells in littermate control (LXRαβflox/flox, n = 9) and LXRαβΔIEC (Villin-Cre × LXRαβflox/flox, n = 7) mice. c, d Representative dot plot (c) and frequencies (d) of Th17 (RORγt+Foxp3−), Treg (RORγt−Foxp3+), and RORγt+ Treg (RORγt+Foxp3+) cells in littermate control (LXRαβflox/flox, n = 15) and LXRαβΔVAV (Vav1-iCre x LXRαβflox/flox, n = 12) mice. Data are represented as means ± SD. ns nonsignificant, *p < 0.05 by unpaired Student’s t test.
Horizontal microbiota transfer from LXRα deficient mice partly restores MLN Th17 cells in WT
Microbiota is considered one of the major drivers of Th17 cell generation in the intestine, as seen by virtually complete absence of Th17 cells in germ-free mice.10 As the lack of LXR in either immune or IEC compartment did not explain the altered Th17 cell frequency, as seen in LXRα−/− mice, we reasoned that alternative factors, such as the microbiota, might cause this expansion. To address the bacterial contribution to MLN Th17 cell generation in WT and LXRα−/− mice by horizontal and/or vertical transfer of bacteria to suckling or adult mice, we performed cross-fostering and co-housing experiments. To evaluate whether bacterial shaping early in life during the lactating period could affect MLN Th17 cells in adulthood, we performed a cross-fostering experiment where newborn WT and LXRα−/− pups from homozygous parents (same provider) were cross-fostered at birth with LXRα−/− and WT dams respectively (Fig. 4a). Four weeks after birth, mice were weaned into separate cages based on their genotypes and MLN T cell composition was analyzed around 8–12 weeks after birth (Fig. 4a). WT mice displayed decreased frequencies of Th17 cells in the MLN compared to LXRα−/− mice regardless of fostering conditions, thus suggesting that the microbiota or nutrients transferred vertically by breastfeeding were not causative of the observed expansion of Th17 cells in LXRα−/− mice (Fig. 4b). Next, to investigate whether horizontal transfer of the microbiota during adulthood might account for Th17 expansion, WT and LXRα−/− non-littermate mice from the same provider were either co-housed or single housed according to their genotype for 4 weeks (Fig. 4c) to allow for normalization of microbiota.23 Of note, WT mice co-housed with LXRα−/− mice partially gained higher frequencies of MLN Th17 cells compared to single housed WT mice. However, similar to what was observed in cross fostered mice, Th17 cells were proportionally higher in the MLN of LXRα−/− mice compared to WT regardless of housing conditions (Fig. 4d, e). Altogether, these data suggest that a distinctive horizontally transferred gut microbiota in LXRα−/− mice partly induce the expansion of Th17 cells in the MLN.
a Schematic representation of the cross-fostering experiment: WT and LXRα−/− newborn pups (non-littermates from the same vendor, Taconic) were either left with their biological mothers or they were swapped between their mothers (foster mother) right after birth and for the entire lactating period. Four weeks after birth, mice were weaned and kept in separate cages based on genotype and fostering mother. Mice were then sacrificed at 8–12 weeks of age and MLN was analyzed by flow cytometry. b Frequencies of Th17 and Treg cells out of CD4 T cells in the MLN of WT and LXRα−/− mice stratified based on genotype and foster mother (each dot represents one mouse out of two independent experiments). c Schematic representation of the co-housing experiment: WT and LXRα−/− mice (non-littermates) coming from different breeding colonies at Taconic were either housed separately (single-housed) based on their genotype or together (co-housed) in the same cage for a period of 4 weeks. Representative dot plots (d) and quantification of frequencies (e) of Th17 and Treg cells in the MLN of WT and LXRα−/− mice either single- or co-housed (each dot represents one mouse out of four independent experiments). Data are represented as means ± SEM. ns nonsignificant, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 by one-way ANOVA with Bonferroni’s post-test.
LXRα mediated regulation of MLN Th17 is independent of antibiotic-sensitive intestinal microbiota
Since the presence of SFB is sufficient to induce Th17 cell priming shortly after colonization,10,24 we evaluated if SFB levels correlate with Th17 frequencies in WT and LXRα−/− non-littermate mice from the same provider. Increased frequency of Th17 cells in the MLN of LXRα−/− mice did not correlate with SFB abundance (Fig. 5a), suggesting that Th17 expansion in LXRα-deficient mice is SFB-independent. To gain a better understanding of the contribution of microbiota in LXRα mediated Th17 regulation, we established heterozygous breeding colonies in our own animal facility. As per comparison with our previous data using mice from Taconic, we also established homozygous (i.e., WT × WT and LXRα−/− × LXRα−/−) breeding colonies. Analysis of LXRα−/− mice established in our animal facility confirmed the increased in Th17 compared to controls (littermates and non-littermates) animals (Supplementary Fig. 6a). Interestingly, this comparison revealed that the difference in Th17 cells between LXRα−/− and control animals was reduced in our animal facility compared to Taconic (Supplementary Fig. 6a), suggesting that the penetrance of the LXRα-mediated Th17 phenotype was animal facility-dependent. Next, we tested if bacterial communities were different between LXRα−/− mice and littermate controls and thus potentially explain the expansion of MLN Th17. Toward this we performed 16S bacterial rRNA sequencing of LXRα−/− mice and littermate controls (Fig. 5b). Of note, we observed significantly higher enrichment of bacterial species belonging to the family Ruminococcaceae and Lachnospiraceae in LXRα−/− mice compared to littermate controls (Fig. 5b, f, Supplementary Fig. 6b, c), indicating that LXRα modulates the composition of the intestinal microbiota. To further test if the distinct microbiota composition accounts for the differences in MLN Th17 cells, we treated LXRα−/− and control mice for 10 days with a broad-spectrum antibiotic cocktail. As expected, we observed a dramatic change in the microbiota composition, which was dominated by Enterobacteriaceae in both LXRα−/− mice and littermate controls (Fig. 5c) and significant abrogation of majority of bacteria in the intestinal tract (Fig. 5d; Supplementary Fig. 6d, e), including SFB (Fig. 5e), Ruminococcaceae and Lachnospiraceae (Fig. 5f). Having demonstrated that the antibiotic treatment depleted the majority of the intestinal microbiota in the LXRα-deficient background, we analyzed the levels of MLN Th17 cells. While the antibiotic treatment resulted in reduction of MLN Th17 cells in control mice, it did not alter Th17 frequencies in LXRα−/− mice (Fig. 5g), suggesting that the expansion of MLN Th17 in LXRα−/− mice was independent of antibiotic-sensitive microbiota. Taken together, our results demonstrate that LXRα regulates MLN Th17 expansion in a microbiota-independent manner.
a Correlation between Th17 frequencies in the MLN and SFB relative amount in the stool samples from WT and LXRα−/− non-littermate mice. b, c Relative abundance of indicated bacterial families in the stool samples from LXRα−/− mice and littermate controls in steady state (b) and after antibiotic (Abx) treatment (c) as determined by 16S rRNA sequencing. d Relative amount of total bacteria per mg of stool in LXRα−/− mice and littermate controls before and after Abx treatment. e Relative amount of SFB (normalized to universal 16S) in LXRα−/− mice and littermate controls before and after Abx treatment. f Plot showing normalized count of Ruminococcaceae and Lachnospiraceae families obtained from 16S rRNA sequencing in LXRα−/− mice and littermate controls before and after Abx treatment (one-tailed unpaired Student’s t test). g Frequencies of Th17 in the MLN of controls (n = 9 untreated, n = 9 Abx treated) and LXRα−/− (n = 10 untreated, n = 11 Abx treated) mice treated or not with antibiotics. Each dot represents one mouse and data are represented as means ± SEM. Closed circles show mice (non-littermates) from Taconic; open circles show mice (littermates) from our animal facility. ns nonsignificant, *p < 0.05, **p < 0.01, ***p < 0.001 by unpaired Student’s t test.
Discussion
LXR has been shown to play an anti-inflammatory role by dampening the differentiation of Th17 cells in a T cell intrinsic manner. Here, using mice lacking LXR in different cell compartments, we have defined a model in which LXR signaling controls the generation of two different T helper cells by distinct mechanisms. While RORγt+ Treg cells are controlled by LXR signaling in CD11c+ myeloid cells, LXR deficiency promotes the expansion of MLN Th17 cells in a microbiota-independent fashion.
Th17 cells are important mediators of antibacterial/antifungal immunity and they also play a key role in the pathogenesis of autoimmune/inflammatory diseases. In agreement with the high density of bacteria colonizing the gut mucosa, the intestine harbors the majority of Th17 cells present in our body. Gut-draining lymphoid tissues, including MLN, are the main sites orchestrating the priming of adaptive immune responses to intestinal luminal antigens. Here, we dissected the role of LXR in shaping MLN Th17 and found that while LXR activation caused a decrease in RORγt+ T cells, lack of LXR resulted in expansion of Th17 and RORγt+ Tregs. Previous studies have claimed that LXR-mediated control of Th17 differentiation occurs in a T cell-intrinsic fashion, as shown by in vitro T cell differentiation assays.18,20 However, by using two different in vivo approaches (mixed BM chimera and conditional depletion of LXR in CD4+ T cells), we showed that LXR is not required on T cells to control Th17 differentiation in the MLN. A recent study reported that LXR deficiency and cholesterol accumulation in DCs altered their antigen presenting function, thus affecting priming and expansion of adaptive immune cells.25 Our analysis of MLN DC subsets in mice lacking LXRα, showed higher numbers of CD103+CD11b+ DCs, previously implicated in the induction of intestinal Th17 differentiation.6 Nevertheless, our results show that conditional ablation of LXR signaling in CD11c+ myeloid cells (or in total immune cells) did not result in any alteration in MLN Th17 cells. By contrast, mice lacking LXR in CD11c+ myeloid cells resulted in increased RORγt+ Tregs in the MLN. These findings pose several appealing questions that remain to be answered: is the generation of RORγt+ Tregs dependent on CD103+CD11b+ DCs as shown for Th176? Since LXRβ is expressed by all DC subsets and LXRβ−/− mice showed increased RORγt+ Tregs, is the regulation of RORγt+ Tregs in LXRαβΔCD11c mice dependent on LXRβ expression in DCs? Does LXR imprint DCs with tolerogenic capacities as shown for other NRs?26 Does altered cholesterol metabolism in DCs skew their potential towards the preferential generation of RORγt+ Treg? Are RORγt+ Tregs generated by LXR-deficient DCs functionally comparable to the ones generated by LXR-sufficient DCs? While these questions await experimental validation, our results thus far suggest that LXR signaling is necessary in antigen-presenting cells to restrain the expansion of RORγt+ Treg cells.
The intestinal epithelium is the first cellular line detecting environmental cues in the lumen and transmitting those signals to underlying immune cells in the lamina propria. For example, impaired sensing of dietary-derived retinoic acid, specifically by IECs, results in an underdeveloped immune system.27 In addition, IECs have been implicated in shaping the differentiation and activity of lymphocytes, including Th17 cells.10,28 However, selective LXR deficiency in IECs alone was not sufficient to expand MLN Th17 cells unlike the whole body LXR deficient mice. These findings could potentially be explained by the requirement of LXR either in non-immune/non-epithelial cells or in immune and epithelial cells simultaneously to control MLN Th17.
With the aim of investigating what contributes to the specific expansion of MLN Th17 in LXRα−/− mice, we tested the relative contribution of the microbiota. Cross-fostering and co-housing experiments suggested that only horizontal transfer of bacteria in adulthood was sufficient to partially increase Th17 cells in WT mice, although this did not reach the level of Th17 cells observed in the MLN of LXRα−/− mice. These results propose a model whereby a combination of genetic deficiency of LXR and microbiota composition controls MLN Th17. We thus used littermate and cohoused mice to further reduce any external or LXR-independent modulation of the microbiota. In these experiments, we observed significantly higher enrichment of bacteria belonging to the family Ruminococcaceae and Lachnospiraceae in LXRα−/− mice compared to littermate controls in steady state, suggesting that lack of LXRα allows preferential colonization of specific groups of bacteria. Both Ruminococcaceae and Lachnospiraceae belong to the order Clostridiales and phylum Firmicutes and have been associated with multiple human diseases including IBD29,30,31 and atherosclerosis.32,33 While the abundance of both Ruminococcaceae and Lachnospiraceae are decreased in IBD patients, suggesting a protective role, they have been shown to be associated with pro-atherogenic effects. Since Th17 cells have been shown to play context dependent functions in both IBD34,35 and atherosclerosis,36 future studies will address whether LXRα mediated regulation/restraint of Ruminococcaceae and Lachnospiraceae impact diseases such as IBD and atherosclerosis in a Th17 cell-(in)dependent manner.
SFB are sufficient to induce Th17 cells in the intestine.10 However, in our microbiota analysis we did not observe any difference in the amount of SFB in the colonic stool of WT and LXRα−/− mice as well as no correlation between the Th17 cells in the MLN with the amount of SFB in the stool samples. While the intestine-draining lymph node, i.e., MLN, serves as the site of T effector cell priming by antigen presenting cells such as DCs, Goto et al.37 demonstrated that priming of SFB-induced Th17 happens in the intestinal lamina propria rather than the draining lymph nodes. Therefore, our observation of SFB-independent increase of Th17 in the MLN of LXRα−/− mice is supported by the notion that SFB-mediated Th17 priming happens in loco in the intestinal lamina propria without affecting the Th17 cells observed in the MLN.37 To further confirm if the increased Th17 observed in LXRα−/− mice was due to any other bacterial species, we treated both control and LXRα−/− mice with broad-spectrum antibiotics. While there was a reduction in total bacteria in both LXRα−/− and control mice, we observed a reduction of MLN Th17 only in control mice. Moreover, 16S rDNA sequencing showed no significant differences in microbiota composition between WT and LXRα−/− mice after antibiotic treatment. These findings suggest that while microbiota influences the priming of Th17 in the MLN of WT mice, in LXRα−/− mice it is microbiota-independent. Although bacterial species constitute the bulk of intestinal microbiota, we cannot rule out if the observed increase in MLN Th17 cells in LXRα−/− is dependent on other minor constituents of the microbiota. Toward this, the analysis of MLN Th17 in germ-free mice after fecal transfer from antibiotic-treated LXRα−/− mice will help understanding if antibiotic-resistant bacterial species, fungi, viruses, and protists, etc. drive Th17 expansion in LXRα−/− mice. Moreover, given the increased Th17 survival in LXRα−/− mice, it is possible that depleting the microbiota over a period of 10 days (as in our antibiotic treatment protocol) might not be sufficient to cause a decrease of Th17 in LXRα−/− mice. Further studies with prolonged antibiotic treatment or germ-free animals are therefore necessary to address these questions.
Overall, using multiple approaches and genetic tools, we demonstrated that LXR restrains the expansion of T helper subsets such as Th17 and RORγt+ Tregs by distinct mechanisms in the MLN. Contrary to previous findings,18 we showed that the effect of LXR on MLN Th17 is T cell extrinsic. We further demonstrated that LXR is required neither on immune nor IECs and is independent of antibiotic-sensitive intestinal bacteria, thus suggesting a new mode of regulation of MLN Th17 that might rely on other untested cell types (e.g., stromal or neuronal cells) or a simultaneous requirement on multiple cell types (e.g., immune and epithelial together). On the other hand, RORγt+ Tregs were dependent on LXR signaling on CD11c+ myeloid cells. This demonstrates that, through different modes, LXR regulates RORγt+ T cells in the intestine draining lymph node. These findings can be further explored to understand the functional consequences of such regulation both in intestinal and extra intestinal tissues under different disease conditions such as IBD, EAE, atherosclerosis.
Materials and methods
Mice
Mice, aged 6–20 weeks of C57BL/6J background were used for all experiments. Wild-type (CD45.2 and CD90.2), CD45.1 and CD90.1 were purchased from either Taconic (Taconic, Ry, Denmark) or from mice bred at the Microbiology, Tumor and Cell Biology (MTC), Karolinska Institutet (CD45.1 mice). CD11c-cre, CD4-cre, and Vav1-icre mice were purchased from Jackson Laboratories and bred locally. LXRα−/−, LXRβ−/−, Villin-cre, and LXRαβflox/flox mice were kindly provided by professor Jan-Åke Gustafsson (Karolinska Institutet, Huddinge) and Rosa26LSL-EYFP mice were kindly provided by Taras Kreslavskiy (Karolinska Institutet, Solna). LXRα−/−, LXRβ−/− were either bred in homozygous conditions at Taconic (Denmark) or bred at Karolinska in homozygous or heterozygous conditions. In experiments with littermate controls, LXRα+/+ or LXRα+/− were used as controls. Animals were maintained under specific pathogen-free conditions at Astrid Fagraeus Laboratory (MPV+ animal facility), AKM or MTC animal facility and handled according to protocols approved by the Institutional Animal Care and Use Committee at the Karolinska Institutet (Stockholm, Sweden). Foster experiments were performed at Taconic (Denmark) and weaned mice were shipped to Karolinska Institute for analysis. For LXR activation in vivo, mice were fed with either a control diet or diet formulated with GW3965 (50 mg/kg/day) with AIN 93G diet as the basal diet (SSNIFF, Germany).
Single-cell suspensions and in vitro cultures
Mesenteric lymph node and spleen cells were isolated by smashing the lymph nodes through a 70 µm cell strainer. Red blood cells (spleen) were lysed using ACK buffer. Remaining cells were pelleted for further use. Colonic lamina propria cells were isolated as previously described.38,39 Briefly, colonic tissue was cleaned from fat, opened longitudinally and cut into 1 cm pieces. Tissues were incubated for 30 min at 37 °C in 20 ml HBSS with 5% FCS, 5 mM EDTA, 1 mM DTT and HEPES 15 mM under gentle shaking followed by two washing steps in 20 mL PBS with 5% FCS and EDTA 1 mM at 37 °C and PBS with 1% FCS and 15 mM HEPES at room temperature. Tissues were then digested in 10 ml of serum-free HBSS with Liberase TL (0.15 mg/ml, Roche) and 0.1 mg/ml DNase I (Roche) at 37 °C at 600 rpm for 45 min followed by filtration through a 100 μm cell strainer. A 44–67% Percoll (Sigma Aldrich) separation was then performed to enrich lymphoid cells.
For in vitro T-cell differentiation, splenic CD4+ T cells were enriched by positive selection using the CD4 (L3T4) isolation kit (Miltenyi) following the manufacturer’s instructions. For polarizing experiments CD4+ T cells were stimulated with 1 µg/mL soluble anti-CD3/CD28 in the presence or absence of 5 µg/mL GSK2033 or GW3965 (Sigma) and incubated at 37 °C with 5% CO2 for 5 days. T-cell subsets were analyzed by FACS on day 5. Following polarizing conditions were used: 2 ng/mL IL-2 (Th0); 2 ng/mL IL-2, 30 ng/mL IL-4, and 1.25 μg/mL anti-IFN-γ (Th2); 2 ng/mL IL-2, 3 ng/mL TGF-β, 30 ng/mL IL-6, 30 ng/mL IL-23, 0.625 μg/mL anti-IL-4, and 1.25 μg/mL anti-IFN-γ (Th17); 2 ng/mL IL-2 and 2 ng/mL TGF-β (Treg).
Co-housing
Three to four weeks after birth, mice coming from separate homozygous colonies (either WT or LXRa−/−) at Taconic were weaned based on sex. From week 6 after birth until sacrifice mice were then either left single-housed (i.e., housed with littermates with the same genotype) or co-housed with mice of the opposite genotype for 4 weeks until sacrifice.
Cross-fostering
Breeding cages with two females and one male mouse with the same genotype (i.e., either WT × WT or LXRα−/− × LXRα−/−) were paired. After three weeks, pregnant females were separated and single-housed in new cages. The following week, when pups were born, the litters were either kept in the same cage or swapped and added to cages with foster female of the opposite genotype. Four weeks after birth, pups were weaned into separate cages based on genotype and sex. Mice were then sacrificed for analysis when 10–19 weeks old.
Antibiotic treatment
WT/LXRα+/− and LXRα−/− mice from our homozygous and heterozygous breedings were treated with antibiotic cocktail for 10 consecutive days. The following cocktail was given by oral gavage: ampicillin (1 mg/ml), kanamycin (1 mg/ml), gentamicin (1 mg/ml), metronidazole (1 mg/ml), neomycin (1 mg/ml), and vancomycin (0.5 mg/ml). After antibiotic treatment, mice were sacrificed, and organs and stool samples were collected for subsequent analysis.
Statistical analysis
Statistical analyses were performed with GraphPad Prism (GraphPad Software, Inc., 2005). Comparisons between two groups was performed using Student t test; comparison between more than two groups was performed using one-way ANOVA with Bonferroni’s post-test. In all cases, p values of less than 0.05 were considered significant.
References
Shale, M., Schiering, C. & Powrie, F. CD4(+) T-cell subsets in intestinal inflammation. Immunol. Rev. 252, 164–182 (2013).
Ohnmacht, C. et al. Mucosal immunology. The microbiota regulates type 2 immunity through RORγt+ T cells. Science 349, 989–993 (2015).
Sefik, E. et al. Mucosal immunology. Individual intestinal symbionts induce a distinct population of RORγ+ regulatory T cells. Science 349, 993–997 (2015).
Yang, B. H. et al. Foxp3(+) T cells expressing RORγt represent a stable regulatory T-cell effector lineage with enhanced suppressive capacity during intestinal inflammation. Mucosal Immunol. 9, 444–457 (2016).
Agalioti, T., Villablanca, E. J., Huber, S. & Gagliani, N. Th17 cell plasticity: the role of dendritic cells and molecular mechanisms. J. Autoimmun. https://doi.org/10.1016/j.jaut.2017.12.003 (2018).
Persson, E. K. et al. IRF4 transcription-factor-dependent CD103(+)CD11b(+) dendritic cells drive mucosal T helper 17 cell differentiation. Immunity 38, 958–969 (2013).
Cosorich, I. et al. High frequency of intestinal Th17 cells correlates with microbiota alterations and disease activity in multiple sclerosis. Sci. Adv. 3, e1700492 (2017).
Wilck, N. et al. Salt-responsive gut commensal modulates Th17 axis and disease. Nature 551, 585–589 (2017).
Haghikia, A. et al. Dietary fatty acids directly impact central nervous system autoimmunity via the small intestine. Immunity 43, 817–829 (2015).
Ivanov, I. I. et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 139, 485–498 (2009).
Zelcer, N. & Tontonoz, P. Liver X receptors as integrators of metabolic and inflammatory signaling. J. Clin. Invest. 116, 607–614 (2006).
Diaz, O. E. et al. Retinoic acid induced cytokines are selectively modulated by liver X receptor activation in zebrafish. Reprod. Toxicol. 93, 163–168 (2020).
Villablanca, E. J. et al. Tumor-mediated liver X receptor-alpha activation inhibits CC chemokine receptor-7 expression on dendritic cells and dampens antitumor responses. Nat. Med. 16, 98–105 (2010).
Tavazoie, M. F. et al. LXR/ApoE activation restricts innate immune suppression in cancer. Cell 172, 825–840.e8 (2018).
Walcher, D. et al. LXR activation reduces proinflammatory cytokine expression in human CD4-positive lymphocytes. Arterioscler. Thromb. Vasc. Biol. 26, 1022–1028 (2006).
Herold, M. et al. Liver X receptor activation promotes differentiation of regulatory T cells. PLoS ONE 12, e0184985 (2017).
Takeuchi, H. et al. Retinoid X receptor agonists modulate Foxp3+ regulatory T cell and Th17 cell differentiation with differential dependence on retinoic acid receptor activation. J. Immunol. 191, 3725–3733 (2013).
Cui, G. et al. Liver X receptor (LXR) mediates negative regulation of mouse and human Th17 differentiation. J. Clin. Invest. 121, 658–670 (2011).
Collins, J. L. et al. Identification of a nonsteroidal liver X receptor agonist through parallel array synthesis of tertiary amines. J. Med. Chem. 45, 1963–1966 (2002).
Bensinger, S. J. et al. LXR signaling couples sterol metabolism to proliferation in the acquired immune response. Cell 134, 97–111 (2008).
Xu, H. et al. Transcriptional atlas of intestinal immune cells reveals that neuropeptide alpha-CGRP modulates group 2 innate lymphoid cell responses. Immunity 51, 696–708 e699 (2019).
Caton, M. L., Smith-Raska, M. R. & Reizis, B. Notch-RBP-J signaling controls the homeostasis of CD8- dendritic cells in the spleen. J. Exp. Med. 204, 1653–1664 (2007).
Caruso, R., Ono, M., Bunker, M. E., Nunez, G. & Inohara, N. Dynamic and asymmetric changes of the microbial communities after cohousing in laboratory mice. Cell Rep. 27, 3401–3412, e3403 (2019).
Atarashi, K. et al. Th17 cell induction by adhesion of microbes to intestinal epithelial cells. Cell 163, 367–380 (2015).
Ito, A. et al. Cholesterol accumulation in CD11c+ immune cells is a causal and targetable factor in autoimmune disease. Immunity 45, 1311–1326 (2016).
Villablanca, E. J. et al. beta7 integrins are required to give rise to intestinal mononuclear phagocytes with tolerogenic potential. Gut 63, 1431–1440 (2014).
Jijon, H. B. et al. Correction: intestinal epithelial cell-specific RARalpha depletion results in aberrant epithelial cell homeostasis and underdeveloped immune system. Mucosal Immunol. 12, 580 (2019).
von Moltke, J., Ji, M., Liang, H. E. & Locksley, R. M. Tuft-cell-derived IL-25 regulates an intestinal ILC2-epithelial response circuit. Nature 529, 221–225 (2016).
Nagao-Kitamoto, H. & Kamada, N. Host-microbial cross-talk in inflammatory bowel disease. Immune Netw. 17, 1–12 (2017).
Morgan, X. C. et al. Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol. 13, R79 (2012).
Lo Presti, A. et al. Fecal and mucosal microbiota profiling in irritable bowel syndrome and inflammatory bowel disease. Front. Microbiol. 10, 1655 (2019).
Liu, B. et al. Western diet feeding influences gut microbiota profiles in apoE knockout mice. Lipids Health Dis. 17, 159 (2018).
Ascher, S. & Reinhardt, C. The gut microbiota: an emerging risk factor for cardiovascular and cerebrovascular disease. Eur. J. Immunol. 48, 564–575 (2018).
Moschen, A. R., Tilg, H. & Raine, T. IL-12, IL-23 and IL-17 in IBD: immunobiology and therapeutic targeting. Nat. Rev. Gastroenterol. Hepatol. 16, 185–196 (2019).
Kempski, J., Brockmann, L., Gagliani, N. & Huber, S. Th17 cell and epithelial cell crosstalk during inflammatory bowel disease and carcinogenesis. Front. Immunol. https://doi.org/10.3389/fimmu.2017.01373 (2017).
Taleb, S., Tedgui, A. & Mallat, Z. IL-17 and Th17 cells in atherosclerosis: subtle and contextual roles. Arterioscler Thromb. Vasc. Biol. 35, 258–264 (2015).
Goto, Y. et al. Segmented filamentous bacteria antigens presented by intestinal dendritic cells drive mucosal Th17 cell differentiation. Immunity 40, 594–607 (2014).
Parigi, S. M. et al. Flt3 ligand expands bona fide innate lymphoid cell precursors in vivo. Sci. Rep. 8, 154 (2018).
Villablanca, E. J. et al. MyD88 and retinoic acid signaling pathways interact to modulate gastrointestinal activities of dendritic cells. Gastroenterology 141, 176–185 (2011).
Acknowledgements
We thank members of the Villablanca lab for helpful comments. S.D. was supported by grants from Cancerfonden (CAN 2016/1206). A.F. was supported by a grant from the German research association (D.F.G., 808021). J.A.G. was supported by Robert A. Welch Foundation (E-0004) and the Swedish Research Council. E.J.V. was supported by grants from the Swedish Research Council VR grant K2015-68×-22765-01-6, FORMAS grant No. FR-2016/0005, Cancerfonden (19 0395 Pj), and Wallenberg Academy Fellow (WAF) program.
Author information
Authors and Affiliations
Contributions
S.M.P., S.D., A.F., R.F.C., and C.D. designed and performed most experiments, analyzed the data, and wrote the paper. K.P.J. and Y.H. performed the analysis. L.E., P.A., and J.A.G. provided the mice and feedback. E.J.V. conceived the study, designed the experiments, analyzed the data, and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Parigi, S.M., Das, S., Frede, A. et al. Liver X receptor regulates Th17 and RORγt+ Treg cells by distinct mechanisms. Mucosal Immunol 14, 411–419 (2021). https://doi.org/10.1038/s41385-020-0323-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41385-020-0323-5