Abstract
Type 2 innate lymphoid cells (ILC2s) play a critical role early in the response to infection by helminths and in the development of allergic reactions. ILC2s are also involved in the physiologic regulation of adipose tissue and its metabolic response to cold shock. We find that the metabolic sensor peroxisome proliferator-activated receptor gamma (PPARγ) is highly expressed in ILC2s of the lung and adipose tissue and increases responsiveness to IL-33. In turn, activation of ILC2 by IL-33 leads to increased expression of PPARγ, a prerequisite for proliferation and expression of the effector cytokines IL-5 and IL-13. In contrast, pharmacological inhibition of PPARγ leads to decreased expression of CD36 and fatty acid uptake, a necessary source of energy for ILC2s and of potential ligands for PPARγ. As a consequence, treatment of mice with a PPARγ antagonist reduces the severity of an ILC2-dependent acute airway inflammation. Together, our results demonstrate the critical role of the metabolic sensor PPARγ for the functions of ILC2s.
Similar content being viewed by others
Introduction
Peroxisome proliferator-activated receptor gamma (PPARγ), a ligand-activated transcription factor and metabolic “sensor,” regulates the expression of genes involved in the storage of fatty acids and the metabolism of glucose. PPARγ is required for the differentiation of adipocytes, and its pharmacological inhibition or absence in mutant mice leads to a loss in functional adipocytes, failure to generate adipose tissue even in response to high fat diet, and hyposensitivity to insulin.1 PPARγ is also involved in the regulation of immunity toward type 2 responses. In myeloid cells, it promotes the differentiation of type 2 macrophages2 and the capacity of dendritic cells (DCs) to induce the generation of Th2 cells.3 In lymphoid cells, PPARγ is critical for the generation of Th2 cells in the context of lung allergic inflammation and intestinal helminth infection,4 whereas it inhibits the generation of Th17 cells.5 PPARγ also promotes the accumulation of type 2 (Gata3+) regulatory T cells (Tregs) in adipose tissues where they regulate insulin sensitivity.6
Innate lymphoid cells (ILCs) are the innate counterpart of T cells and mirror the effector cytokine production of Th1, Th2, and Th17 cells.7 Contrary to the time-consuming clonal selection and expansion process that T cells undergo upon antigen recognition, ILCs respond promptly to inducer cytokines expressed by myeloid or non-hematopoietic cells upon infection or injury. Therefore, ILCs play a unique role early in immunity in the regulation of the unfolding immune response. Type 2 innate lymphoid cells (ILC2s) have been first identified in the context of intestinal helminth infection,8,9,10 and further shown to play an essential role in acute lung allergic inflammation.11,12 ILC2s are activated by the inducer cytokines interleukin (IL)-25, IL-33, and TSLP that are expressed by stromal, epithelial, and endothelial cells in response to mechanical stress, structural injury, or chemical cues that report infection by helminths.7,12 ILC2s express the effector cytokines IL-5 and IL-13, which induce the recruitment of eosinophils and the production of mucus by epithelial cells, and prime DCs for the induction of Th2 cells. ILC2s are also involved in the IL-33-mediated beiging of adipose tissue in response to cold shock.13
Even though ILC2s, like PPARγ at the molecular level, play a prominent role in the development of type 2 immune responses, a role of PPARγ in the function of ILC2s has only recently been reported through its role in the acquisition of fatty acids.14 Here, we demonstrate that PPARγ is highly expressed in ILC2s, and is required for their proliferation and effector functions through the control of energy metabolism and the establishment of a positive feedback loop with the sensing of IL-33. As a consequence, pharmacological inhibition of PPARγ blocks the development of an ILC2-dependent acute lung inflammation. Our data reinforce the notion that PPARγ is a metabolic sensor of fatty acids that promotes type 2 immunity both at the innate and the adaptive levels.
Results
ILC2s express high levels of PPARγ in lung and adipose tissue
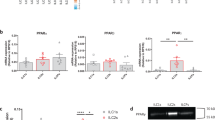
Among ILCs, ILC2s are best characterized by the expression of high levels of the transcription factor Gata3. Therefore, to facilitate the identification of ILC2s, we used heterozygous reporter mice that express EGFP under control of the Gata3 gene locus (Fig. S1A). At the steady state, ILC2s in the lung and (visceral, VAT, and subcutaneous, SC) adipose tissues expressed the highest levels of PPARγ, as compared to ILC3s, B and T cells, and ILC2s in secondary lymphoid tissues (Fig. 1a, b). The expression of PPARγ was further increased in lung and VAT ILC2s and ILC3s after intraperitoneal administration of recombinant IL-33 (Fig. 1c, d). Of note, transcripts for PPARγ, but not for PPARα or PPARβ, were increased following IL-33 treatment (Fig. S1B). Together, these data show that the expression level of PPARγ is highest in ILC2s as compared to other subsets of lymphoid cells, and further increases upon activation.
a Expression of PPARγ in ILC2s, ILC3s, total T and B lymphocytes isolated from lung, visceral adipose tissue (VAT). b Expression of PPARγ (mean fluorescence intensity, MFI) in ILC2s, ILC3s, T and B lymphocytes from lung, VAT and subcutaneous (SC) adipose tissue, lymph nodes (LN) and spleen of Gata3 reporter mice (n = 10 mice, in three independent experiments). c PPARγ expression levels in lung ILC2s from Gata3 reporter mice injected into the peritoneum with IL-33 or PBS and PPARγ-deficient mice. d PPARγ MFI in ILC2s, ILC3s, T and B lymphocytes from lung and VAT of Gata3 reporter mice injected into the peritoneum with IL-33 or PBS. e Frequency of ILC2s in CD45+ cells in lung, VAT, and SC of Gata3 reporter mice treated for 2 weeks with the PPARγ antagonist GW9662 or PBS, or in the lung of PPARγ-deficient mice. f Frequency of ILC2s in CD45+ cells in lung, VAT, and SC of Gata3 reporter mice treated with IL-33 + vehicle, in combination with the antagonist GW9662, or with the agonist rosiglitazone, or from the lung of PPARγ-deficient mice treated with IL-33 only. Data are from two or three independent experiments. Each data point represents an individual mouse. Data represent mean (±SD). Statistical analysis: b, f one-way analysis of variance ANOVA; d, e Mann–Whitney U test. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; ****P ≤ 0.0001. ns not significant.
PPARγ is required for the generation and expansion of tissue-resident ILC2s
In order to assess whether PPARγ is involved in the generation of tissue-resident ILC2s, we examined the presence of ILC2s in lungs and adipose tissues of mice treated with the PPARγ antagonist GW9662, as well as in PPARγ-deficient mice (which also lack adipose tissues). The number and frequency of ILC2s were significantly reduced in mice treated with GW9662, as well as in PPARγ-deficient mice, at the steady state (Figs. 1e and S1C). Furthermore, pharmacological inhibition of PPARγ with GW9662 blocked the IL-33-induced increase in ILC2s in lungs and adipose tissues (Fig. 1f). Similarly, the IL-33-induced increase in lung ILC2s was dramatically reduced in PPARγ-deficient mice (Fig. 1f, right panel). In contrast, rosiglitazone (ROSI), a selective agonist for PPARγ (Fig. S1B), potentiated the increase in ILC2 numbers (Figs. 1f and S1D). These data show that PPARγ is required for the establishment of normal populations of ILC2s in lung and adipose tissues, as well as for their expansion in response to IL-33.
PPARγ is required for ILC2s effector functions
We next assessed whether PPARγ was required for the expression of effector cytokines by ILC2s. The IL-33-induced expression of IL-5 and IL-13 by ILC2s was significantly inhibited in mice treated with GW9662 and in PPARγ-deficient mice (Figs. 2a, b and S2A, B). Interestingly, the frequency of lung ILC2s expressing ST2, a component of the receptor for IL-33, correlated with the mean expression level of PPARγ at the steady state and after IL-33 challenge (Fig. 2c). The expression level of PPARγ also correlated with the frequency of ILC2s expressing IL-5 and IL-13 (Fig. 2d). Together, these correlations suggest a positive feedback loop between IL-33- and PPARγ-activated pathways. In accordance with this hypothesis, chromatin immunoprecipitation and sequencing (ChIP-Seq) data, available on the GEO (GSE115505) and EMBL-EBI (E-MTAB-7255) servers, showed the presence of PPARγ binding sites in promoter and intronic regions of the Il1rl1 gene coding for ST2, in macrophages and in T helper cells polarized by IL-4 (Fig. S3A, B). Furthermore, treatment with GW9662 led to a decrease in the expression of ST2 (Fig. 2e), as well as in Gata3 (Fig. 2f), indicating that PPARγ regulates at least two key factors involved in the generation and function of ILC2s. In order to assess whether PPARγ controls the function of ILC2s in a cell-intrinsic manner, ILC2s were isolated from the lung of Gata3 reporter mice and cultured in the presence of IL-33 and GW9662. In agreement with a cell-intrinsic regulation of ILC2s by PPARγ, GW9662 blocked IL-33-induced expression and production of IL-5 and IL-13 (Fig. 2g, h), whereas ROSI potentiated the effect of IL-33 (Fig. S2C). Moreover, GW9662 prevented the IL-33-induced proliferation of ILC2s, while ROSI synergized with IL-33, thus confirming the key role played by PPARγ in the expansion of ILC2s (Fig. 3a, b).
a Frequency of IL-5+IL-13+, IL-5+, and IL-13+ ILC2 cells from Gata3 reporter mice treated with PBS, IL-33, or IL-33 and the PPARγ antagonist GW9662 (data from three independent experiments). b Frequency of IL-5+IL-13+ ILC2 cells in PPARγ-deficient mice treated with PBS or IL-33. Correlation between MFI of PPARγ expression and frequency of ST2+ cells (c) or frequency of IL-5+IL-13+ ILC2 cells (d) from lung and VAT of mice treated with PBS or IL-33. MFI of ST2 expression (e) and of Gata3 expression (f) in lung and adipose tissue ILC2s from Gata3 reporter mice treated with PBS, IL-33, or IL-33 and the PPARγ antagonist GW9662. Intracellular cytokine staining for IL-5 and IL-13 in ILC2s (g) sorted from the lungs of Gata3 reporter mice, and (h) production of these cytokines after 72 h of cell culture treated with PBS, IL-33, or IL-33 and GW9662. Data are from one or two independent experiments. Each data point represents an individual mouse. Data represent mean (±SD). Statistical analysis: a, b, e, f one-way analysis of variance ANOVA; g, h Mann–Whitney U test; c, d Spearman’s rank test was used to determine correlations. *P ≤ 0.05; **P ≤ 0.01; ****P ≤ 0.0001. ns not significant.
a Proliferation measured by dilution in VPD fluorescence intensity by ILC2s isolated form Gata3 reporter mice and cultured in plain medium (NS), or in the presence of IL-33 or IL-33 and GW9662. Representative overlay FACS plot (left panel) and percentage of number of cell division (D0, D1, D2, D3) (right panel). b Proliferation index (PI) measured after 72 h of culture (Ratio of VPD MFI for each condition by VPD MFI at day 0). c Expression of Ptgs2 and Alox5 by lymphoid cells, from the RNA Seq Immgen database. Proliferation of (d) and cytokine expression by (e) ILC2s isolated form Gata3 reporter mice and cultured in plain medium (NS), or in the presence of IL-33, or IL-33 and the cyclooxygenase inhibitor Diclofenac, the inhibitor of 5-lipoxygenase activating protein Bay-X-1005, or the PPARγ agonist rosiglitazone (left panel) and representative cytometry dot plot (right panel). Levels of fatty acids uptake, as measured by incorporation of bodipy FLC16, in ILC2s isolated from lung and VAT of Gata3 reporter mice treated with PBS, IL-33, or IL-33 and GW9662 (f) and in ILC2s isolated from the lung of PPARγ-deficient mice treated with PBS or IL-33 (g). h Expression of CD36 in ILC2s from lung and VAT of Gata3 reporter mice treated with IL-33 or IL-33 and GW9662. Glucose uptake measured by 2NBDG incorporation, in ILC2s isolated from lung and VAT of Gata3 reporter mice treated with PBS, IL-33, or IL-33 and GW9662 (i) and in ILC2s isolated from the lung of PPARγ-deficient mice treated with PBS or IL-33 (j). Data are from one or two independent experiments. Each data point represents an individual mouse. Data represent mean (±SD). Statistical analysis: b, d Mann–Whitney U test; f–j one-way analysis of variance ANOVA. *P ≤ 0.05; **P ≤ 0.01; ****P ≤ 0.0001. ns not significant.
ILC2s may generate eicosanoid ligands of PPARγ
The inhibition of effector functions and proliferation by a PPARγ antagonist, in cultures of FACS-sorted ILC2s, suggests that these cells produce PPARγ ligands. In accordance with the Immgen database (www.immgen.org), genes involved in the production of eicosanoid ligands of PPARγ, such as prostaglandin-endoperoxide synthase 2 (or cyclooxygenase 2, Ptgs2) and arachidonate 5-lipoxygenase (Alox5), were expressed at higher levels in ILC2s as compared to the other ILC subsets (Fig. 3c). We therefore investigated the functional relevance of the expression of these enzymes in ILC2s. To this end, ILC2s were cultured in the presence of IL- 33, as well as of the cyclooxygenase inhibitor, diclofenac, or an inhibitor of 5-lipoxygenase activating protein, Bay-X-1005. Both inhibitors blocked IL-33-induced proliferation of ILC2s as well as IL-5 and IL-13 production, indicating that ILC2s may produce their own eicosanoid PPARγ ligands (Fig. 3d, e).
PPARγ controls the energy metabolism of ILC2s
Fatty acids are required by ILC2s for the expression of effector cytokines in the context of helminth infection and lung allergy.14,15 As PPARγ regulates the uptake and storage of fatty acids,16 it appears thereby to control the effector functions of ILC2s.14 In accordance with this view, ILC2s from mice treated with IL-33 and GW9662 (Figs. 3f and S4A), or deficient in PPARγ (Fig. 3g), showed significantly lower levels of fatty acids uptake as compared to ILC2s carrying normal PPARγ activity. This deficit in fatty acid uptake correlated with a reduced expression by ILC2s of the fatty acid transporter CD36 in mice treated with GW9662 (Fig. 3h). Confirming the role of fatty acid uptake in ILC2s activity, CD36 inhibition by sulfosuccinimidyl oleate (SSO) significantly decreased IL-33-induced expansion (Figs. 4a and S5A) and cytokine production (Fig. 4b) of lung and VAT ILC2s. Furthermore, pharmacological blockade of CD36 resulted in decreased expression of PPARγ and CD36 itself in ILC2 cells (Fig. 4c, d), whereas such treatment had no significant effect on the levels of PPARγ in T and B cells (Fig. S5B, C). Altogether, these data indicate that PPARγ controls the uptake of fatty acids, at least in part, via the lipid transporter CD36. In turn, CD36 may transport particular lipids into ILC2s that can be converted into PPARγ ligands (Fig. 3d, e). Inhibition or absence of PPARγ also reduced the levels of glucose uptake (Figs. 3i, j and S4B), a likely consequence of the modulation by PPARγ of glucose transporters expression.17
Frequency of ILC2s in CD45+ cells (a) and of IL-5+IL-13+ ILC2 cells (b) in lung and VAT tissues of Gata3 reporter mice treated with the CD36 inhibitor sulfosuccinimidyl oleate (SSO) and IL-33. Expression of PPARγ (mean fluorescence intensity, MFI) (c) and CD36 (d) in ILC2s isolated from lung and VAT of Gata3 reporter mice treated with vehicle or different combinations of SSO and IL-33. Each data point represents an individual mouse. Data represent mean (±SD). Statistical analysis: a–d one-way ANOVA analysis of variance; *P ≤ 0.05; ** P ≤ 0.01; ***P ≤ 0.001; ****P ≤ 0.0001. ns not significant.
Prevention of acute allergy by pharmacological inhibition of PPARγ
As PPARγ controls the expansion and effector functions of ILC2s, we assessed whether pharmacological inhibition of PPARγ was effective in the prevention of acute airway inflammation induced by papain, shown previously to depend on ILC2s18 (Fig. S6A). In this context, PPARγ expression was increased in ILC2s and to a lesser degree in ILC3s, but not in B and T cells (Fig. 5a). In Gata3 reporter mice treated with both papain and GW9662, the frequencies of ILC2s and their production of IL-5 and IL-13 were reduced in the lung, as expected (Figs. 5b, c and S6B). Furthermore, PPARγ expression correlated with the frequency of ST2+ ILC2 in the lung of papain challenged mice (Fig. 5d). Measurements of lipid and glucose uptakes in ILC2s indicated that lung inflammation induced by papain drove metabolic changes similar to that observed with IL-33 challenge, changes that were reverted by GW9662 treatment (Fig. 5e, f). As a consequence of GW9662 treatment, the number of eosinophils, the main mediators of the pro-allergic airway response that are recruited by IL-5, were decreased tenfold in the lung of GW9662-treated mice (Figs. 5g and S6C). The number of lung macrophages was also reduced, while neutrophils were not (Figs. 5h, i and S6D, E). Of note, alveolar macrophages express high levels of PPARγ and are therefore likely to be directly affected by GW9662.19,20 Finally, a decrease in IL-5 and IL-13 secretion was observed in the BAL fluid from mice treated with GW9662 after papain challenge (Fig. S6F).
a PPARγ expression in ILC2s, ILC3s, and total T and B lymphocytes isolated from the lung of Gata3 reporter mice challenged with PBS or papain. Frequency of (b) and cytokine expression by (c) ILC2s in the lung of mice treated with PBS, papain, GW9662, or a combination of papain and GW9662. d Correlation between PPARγ expression and frequency of ST2+ cells from the lung of Gata3 reporter mice treated with PBS or Papain. e Levels of fatty acids uptake, as measured by incorporation of bodipy FLC16 in lung ILC2s of mice treated with PBS, papain, GW9662, or a combination of papain and GW9662. f ILC2 glucose uptake measured by 2NBDG incorporation in lung ILC2s of mice treated with PBS, papain, GW9662, or a combination of papain and GW9662. g–i Absolute numbers of eosinophils, macrophages, and neutrophils in the lungs of mice as in (b). Representative data are from two or three independent experiments. Each data point represents an individual mouse. Data represent mean (±SD). Statistical analysis: a Mann–Whitney U test; b–h one-way analysis of variance ANOVA; d Spearman’s rank test was used to determine correlations. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001. ns not significant.
Altogether, our results demonstrate that PPARγ is a critical regulator of ILC2 activity through the control of Gata3 and ST2 expression levels, as well as of CD36 and fatty acid uptake. Therefore, PPARγ may be targeted to modulate the activity of ILC2s during allergic inflammation and thereby preventing or reducing the severity of allergy.
Discussion
Our data demonstrate that PPARγ directly controls the effector functions of ILC2s. Among ILCs, ILC2s expressed the highest level of PPARγ, which was further increased upon activation by the type 2 inducer cytokine IL-33. Inhibition of PPARγ, or its absence in mutant mice, led to decreased numbers of ILC2s in tissues, decreased expression of Gata3 (a critical transcription factor for the differentiation and function of ILC2s), decreased expression of the IL-33 receptor, and a consequent decrease in the expression of the effector cytokines IL-5 and IL-13. This effect of PPARγ on ILC2s was cell intrinsic as it was recapitulated in cultures of isolated ILC2s, in accordance with the hypothesis that ILC2s may import fatty acid via CD36 and manufacture eicosanoids ligands for PPARγ. Finally, pharmacological inhibition of PPARγ modulated the acute allergic airway inflammation induced by papain, an inflammation that is dependent on the activation of ILC2s.11,12
These data are in agreement with earlier results showing that PPARγ is involved in different elements of type 2 immunity. In particular, PPARγ is required for the generation and effector functions of Th2 cells in the context of lung allergy and helminth infection by Heligmosomoides polygyrus.4 PPARγ is also required for the accumulation of type 2 Tregs in VAT, which contribute to maintain VAT insulin sensitivity,6 as well as in the generation and maintenance of type 2 macrophages (M2) while antagonizing type 1 macrophages (M1) (for a review on the role of PPARγ in macrophage polarization see ref. 21). In contrast, the activation of PPARγ inhibits the generation of Th17 cells,5 in accordance with a broad role for PPARγ in the promotion of type 2 immune responses and inhibition of competing responses.
We have observed that the frequency of ILC2s expressing ST2, as well as the frequency of ILC2s expressing IL-5 and IL-13, was correlated with the mean expression level of PPARγ. In addition, the expression level of Gata3 was decreased in the absence of PPARγ or in the presence of a PPARγ inhibitor. Together, this indicates that PPARγ, a transcription factor, is directly or indirectly involved in the regulation of the genes coding for ST2 and Gata3. In support of this view, CHIP-seq data obtained with polarized M2 macrophages and Th2 cells show that PPARγ binds to promoter and intronic regions of Il1rl1 (coding for ST2)22,23 (Fig. S3). PPARγ also regulates gene expression by affecting the epigenetic code and local chromatin accessibility. In the process of macrophage polarization by IL-4, PPARγ facilitates the expression of a network of genes involved in extracellular matrix remodeling through recruitment of the coactivator P300 and the DNA repair protein RAD21.22 It will therefore be interesting to investigate whether such mechanisms operate in the regulation of ILC2 differentiation and activity by PPARγ.
We observed that the treatment of isolated ILC2s with a PPARγ antagonist affected their proliferation and function, suggesting that ILC2s produce PPARγ ligands, such as eicosanoids. Interestingly, according to the Immgen database, ILC2s express several genes coding for enzymes involved in the manufacture of eicosanoids, such as Ptgs2 and Alox5. We found that inhibition of these enzymes decreased the activation of ILC2s, suggesting that PPARγ, in ILC2s, is activated in a cell-intrinsic fashion. Furthermore, the inhibition of CD36, a fatty acid transporter expressed on the cell membrane, resulted in the decreased expression of PPARγ and decreased activity of ILC2s, in accordance with the hypothesis that CD36 also transports lipid precursors of PPARγ ligands into ILC2s. These findings shed a new light on the potential mechanisms involved in the efficacy of leukotriene neutralization for the treatment of asthma.24 It will therefore be interesting to assess whether the expression and activity of Ptgs2 and Alox5 are augmented by IL-33, and to isolate PPARγ ligands produced by ILC2s. Nevertheless, it is also possible that the activity of PPARγ in ILC2s is induced by pairing with the nuclear receptor RXR, and therefore, is ligand-independent yet sensitive to PPARγ antagonists.22
A common trait of ILC2s, Th2, VAT Tregs, and type 2 macrophages is their reliance on fatty acid oxidation as a source of energy.25 To this end, uptake of triacylglycerol via CD36 and the subsequent elevation in oxidative phosphorylation are critical steps in the generation of M2 macrophages.26 Similarly, we find that ILC2s activated by IL-33 upregulate their expression of CD36 and increase their storage of fatty acids. As reported earlier in macrophages and hepatocytes, our data demonstrate that PPARγ is required for CD36 expression and fatty acid storage, which also leads to increased cellular levels of glucose. The storage of lipids into cytoplasmic droplets, a PPARγ-dependent mechanism, has recently been shown to be necessary for proper function of ILC2s. However, the role of these lipid droplets remains to be elucidated.14 It remains also to be understood whether fatty acid uptake and oxidative phosphorylation are driving a type 2 immune program, or whether PPARγ orchestrates these metabolic and immune changes independently. Therefore, elucidating the contribution of PPARγ to ILC2 biology in additional models of ILC2 activation (such as helminth infection or bleomycin-induced lung injury) will be informative. Further studies are also required to elucidate how IL-33 increases PPARγ expression. Our data suggest, but do not demonstrate, that IL-33 directly regulates PPARγ expression.
In summary, we show here that PPARγ is a central regulator of ILC2 function through the modulation of proliferation and cytokine production pathways, as well as through regulation of energy metabolism. Thus, PPARγ is a potential target for the modulation of ILC2 activity in order to mitigate acute allergic reactions, or, on the contrary, to boost ILC2 function in the context of helminth infection. Given the association of PPARγ with fatty acid storage and energy metabolism, our results also support the notion that management of lipid intake, through tailored diets, for example, is a potential avenue to modulate ILC2 activity in particular, and type 2 immunity in general.
Materials and methods
Mice
C57BL/6 mice were purchased from Charles River and exposed to the environment of the local animal facility for at Institut Pasteur for at least for 2 weeks before mating. Gata3-GFP reporter mice were generated by Meinrad Busslinger,27 bred at Institut Pasteur, and used in all experiments as heterozygotes. PPARγ-deficient mice were generated by breeding Sox-2Cre and PPARγ-floxed mice as described in.28 Experiments were performed on 8–9 weeks old animals of both genders. All animal experiments were approved by the committee on animal experimentation of the Institut Pasteur and by the French Ministry of Research.
In vivo treatments
Mice were treated with IL-33 injected into the peritoneum (10 µg/kg/d) and/or GW9662 (10 mg/kg/d) and/or ROSI (10 mg/kg/d) and/or SSO (10 mg/kg/d) for 1 week (single treatment) or 2 weeks (double treatment). For the experimental model of acute airway inflammation, mice were exposed, under anesthesia, to intranasal inhalation of papain every 3 days (150 µg in PBS per mouse, Wako Pure Chemical, >0.5 units/g), by placing a drop in the nasal cavity of the mouse, for a total of five challenges, and then treated with GW9662 injected into the peritoneum (10 mg/kg/d) for 1 more week.
Flow cytometry
Lungs and adipose tissues were minced and digested with Liberase-TM and Liberase-TL, respectively (Roche) and DNase (Roche) for 1 h at 37 °C. After filtration through a nylon mesh (40 µm cell strainer; Greiner), single cells were collected. Cells were stained for 10 min at 4 °C with LIVE/DEAD fixable blue dead stain kit (Invitrogen). Cells were further incubated with anti-mouse CD16/CD32 mAb (93; eBioscience) for FcR blocking for 15 min on ice and stained for 20 min with antibodies to surface markers. For intracellular staining, cells were fixed and permeabilized with a commercially available fixation/permeabilization kit (eBioscience). Intracellular staining was performed with antibodies specific for Gata3 (FITC), RORγt (PE), or PPARγ (APC) (validated in PPARγKO mice) or for IL-5 (BV421) or IL-13 (PE). Briefly, cells were stimulated or not with 50 ng/ml PMA and 500 ng/ml ionomycin for 4 h in RPMI 1640 (Invitrogen) containing 10% FCS, 25 mM HEPES, 0.05 mM 2-mercaptoethanol, and 100 U/ml of penicillin and streptomycin. For measurements of glucose and fatty acid uptake, cells were washed in PBS and incubated in 100 µM of 2-(n-(7- nitrobenzo-2-oxa-1,3-diazol-4-yl)amino)-2-deoxyglucose (Invitrogen) or in 10 µM of BODIPY™ FLC16 probe for 20 min at 37 °C. Cells were then washed with cold PBS and stained with surface markers. All stainings were analyzed on an LSR Fortessa flow cytometer (Becton Dickinson) and data analyzed using FlowJo v10.6 (Tree Star, Inc.).
In vitro ILC2 culture assays
ILC2s were sorted from Gata3 reporter mice (using a BD FACS ARIA III) using a gate on live Gata3-GFP+ CD45+CD19−CD3−CD127+ST2+ cells and directly plated into 96-well plate at a density of 5000 cells per well. Cultures were maintained for 72 h in complete RPMI 1640 (Invitrogen) containing 10% FCS, 25 mM HEPES, 0.05 mM 2-mercaptoethanol, 100 U/ml penicillin and streptomycin, and 10 ng/ml of rIL-2 and rIL-7 (R&D system). The following molecules were then added in the different experiments: rIL-33 at 10 ng/ml (Biolegend); PPARγ antagonist GW9662 (10 µM); Cox inhibitor, Diclofenac at 1.5 µM; Lox inhibitor, Bay-X-1005 at 100 nM, and PPARγ agonist ROSI at 10 µM. For proliferation assays, sorted ILC2 cells were first stained with violet proliferation dye (VPD) (BD Bioscience) following manufacturer instructions and seeded into 96-well plate.
Elisa
Cytokines (IL-5 and IL-13) were measured by ELISAs according to the manufacturers’ recommendations (R&D System).
Quantitative PCR
Sorted ILC2s were immediately stored in Lysing solution from Single Cell RNA Purification Kit (NORGEN) followed by RNA isolation. cDNA synthesis and purification were performed using the reverse transcriptase SuperScript IV kit (Invitrogen). Real-time quantitative PCR cDNA was performed using the SybrGreen Mix and primers from Biorad. Ct values were normalized to the mean Ct obtained with the two housekeeping genes Gapdh, and Hprt in each sample.
Statistical analysis
Graph generation and statistical analysis were performed using GraphPad Prism software (GraphPad Software, San Diego, California, USA). Each data point represents an individual mouse or in vitro replicates. Error bars show that SD and p values were calculated using the tests indicated in figure legends.
References
Gilardi, F. et al. Systemic PPARγ deletion in mice provokes lipoatrophy, organomegaly, severe type 2 diabetes and metabolic inflexibility. Metab. Clin. Exp. 95, 8–20 (2019).
Bouhlel, M. A. et al. PPARgamma activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. 6, 137–143 https://doi.org/10.1016/j.cmet.2007.06.010 (2007).
Nobs, S. P. et al. PPARγ in dendritic cells and T cells drives pathogenic type-2 effector responses in lung inflammation. J. Exp. Med. 214, 3015–3035 (2017).
Chen, T. et al. PPAR-γ promotes type 2 immune responses in allergy and nematode infection. Sci. Immunol. 2, eaal5196 (2017).
Klotz, L. et al. The nuclear receptor PPARγ selectively inhibits Th17 differentiation in a T cell–intrinsic fashion and suppresses CNS autoimmunity. J. Exp. Med. 206, 2079–2089 (2009).
Cipolletta, D. et al. PPAR-γ is a major driver of the accumulation and phenotype of adipose tissue Treg cells. 486, 549–553 https://doi.org/doi:10.1038/nature11132 (2012).
Eberl, G. et al. Innate lymphoid cells. Innate lymphoid cells: a new paradigm in immunology. Science 348, aaa6566 (2015).
Moro, K. et al. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature 463, 540–544 (2010).
Neill, D. R. et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature 464, 1367–1370 (2010).
Price, A. E. et al. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc. Natl Acad. Sci. USA 107, 11489–11494 (2010).
Halim, T. Y. F. et al. Group 2 innate lymphoid cells are critical for the initiation of adaptive T helper 2 cell-mediated allergic lung inflammation. Immunity 40, 425–435 (2014).
McKenzie, A. N. J., Spits, H. & Eberl, G. Innate lymphoid cells in inflammation and immunity. Immunity 41, 366–374 (2014).
Lee, M. -W. et al. Activated type 2 innate lymphoid cells regulate beige fat biogenesis. Cell 160, 74–87 (2015).
Karagiannis, F. et al. Lipid-droplet formation drives pathogenic group 2 innate lymphoid cells in airway inflammation. Immunity 52, 620–634.e6 (2020).
Wilhelm, C. et al. Critical role of fatty acid metabolism in ILC2-mediated barrier protection during malnutrition and helminth infection. J. Exp. Med. 213, 1409–1418 (2016).
Lee, Y. K., Park, J. E., Lee, M. & Hardwick, J. P. Hepatic lipid homeostasis by peroxisome proliferator-activated receptor gamma 2. Liver Res. 2, 209–215 (2018).
Liao, W. et al. Suppression of PPARγ attenuates insulin-stimulated glucose uptake by affecting both GLUT1 and GLUT4 in 3T3-L1 adipocytes. Am. J. Physiol. Endocrinol. Metab. 293, E219–27 (2007).
Verhoef, P. A. et al. Intrinsic functional defects of type 2 innate lymphoid cells impair innate allergic inflammation in promyelocytic leukemia zinc finger (PLZF)-deficient mice. J. Allergy Clin. Immunol. 137, 591–600.e1 (2016).
Schneider, C. et al. Induction of the nuclear receptor PPAR-γ by the cytokine GM-CSF is critical for the differentiation of fetal monocytes into alveolar macrophages. Nat. Immunol. 15, 1026–1037 (2014).
Ginhoux, F. Fate PPAR-titioning: PPAR-γ “instructs” alveolar macrophage development. Nat. Immunol. 15, 1005–1007 (2014).
Olefsky, J. M. & Glass, C. K. Macrophages, inflammation, and insulin resistance. Annu. Rev. Physiol. 72, 219–246 (2010).
Daniel, B. et al. The nuclear receptor PPARγ controls progressive macrophage polarization as a ligand-insensitive epigenomic ratchet of transcriptional memory. Immunity 49, 615–626.e6 (2018).
Henriksson, J. et al. Genome-wide CRISPR Screens in T Helper Cells Reveal Pervasive Crosstalk between Activation and Differentiation. Cell 176, 882–896.e18 (2019)
Claesson, H. E. & Dahlén, S. E. Asthma and leukotrienes: antileukotrienes as novel anti-asthmatic drugs. J. Intern. Med. 245, 205–227 (1999).
Pelgrom, L. R. & Everts, B. Metabolic control of type 2 immunity. Eur. J. Immunol. 47, 1266–1275 (2017).
Huang, S. C. -C. et al. Cell-intrinsic lysosomal lipolysis is essential for alternative activation of macrophages. Nat. Immunol. 15, 846–855 (2014).
Grote, D., Souabni, A., Busslinger, M. & Bouchard, M. Pax 2/8-regulated Gata 3 expression is necessary for morphogenesis and guidance of the nephric duct in the developing kidney. Development 133, 53–61 (2006).
Nadra, K. et al. PPARgamma in placental angiogenesis. Endocrinology 151, 4969–4981 (2010).
Acknowledgements
We thank all members of the Microenvironment and Immunity unit for discussion and support. This work was supported by Institut Pasteur, INSERM, grant DEQ20160334871 from the Fondation pour la Recherche Médicale, and by the Fondation Arthritis. T.A. was supported by fellowships from EMBO and the Israel National Postdoctoral Award Program for Advancing Women in Science, M.F. by a fellowship from French PIA project “Lorraine Université d’Excellence,” ANR-15-IDEX-04-LUE, and M.B. by Boehringer Ingelheim.
Author information
Authors and Affiliations
Contributions
G.E. and D.M. designed the study and supervised the project; T.F. and T.A. designed parts of the study and performed most of the experiments with the help of M.F., J.Y.J., and M.B. generated and provided Gata3 reporter mice. All authors were involved in writing the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Fali, T., Aychek, T., Ferhat, M. et al. Metabolic regulation by PPARγ is required for IL-33-mediated activation of ILC2s in lung and adipose tissue. Mucosal Immunol 14, 585–593 (2021). https://doi.org/10.1038/s41385-020-00351-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41385-020-00351-w
This article is cited by
-
The modulation of pulmonary group 2 innate lymphoid cell function in asthma: from inflammatory mediators to environmental and metabolic factors
Experimental & Molecular Medicine (2023)
-
Immune Metabolism in TH2 Responses: New Opportunities to Improve Allergy Treatment — Cell Type-Specific Findings (Part 2)
Current Allergy and Asthma Reports (2023)
-
Resident and migratory adipose immune cells control systemic metabolism and thermogenesis
Cellular & Molecular Immunology (2022)
-
Food for thought – ILC metabolism in the context of helminth infections
Mucosal Immunology (2022)
-
NLRP3 priming due to skin damage precedes LTP allergic sensitization in a mouse model
Scientific Reports (2022)