Abstract
Current acellular pertussis (aP) vaccines induce strong antibody and Th2 responses but fail to protect against nasal colonization and transmission of Bordetella pertussis. Furthermore, immunity wanes rapidly after immunization. We have developed a novel adjuvant combination (called LP-GMP), comprising c-di-GMP, an intracellular receptor stimulator of interferon genes (STING) agonist, and LP1569, a TLR2 agonist from B. pertussis, which synergistically induces production of IFN-β, IL-12 and IL-23, and maturation of dendritic cells. Parenteral immunization of mice with an experimental aP vaccine formulated with LP-GMP promoted Th1 and Th17 responses and conferred protection against lung infection with B. pertussis. Intranasal immunization with the same aP vaccine-induced potent B. pertussis-specific Th17 responses and IL-17-secreting respiratory tissue-resident memory (TRM) CD4 T cells, and conferred a high level of protection against nasal colonization as well as lung infection, which was sustained for at least 10 months. Furthermore, long-term protection against nasal colonization with B. pertussis correlated with the number of IL-17-secreting TRM cells in nasal tissue. Our study has identified an approach for inducing IL-17-secreting TRM cells that sustain sterilizing immunity against nasal colonization of mice with B. pertussis, and could form the basis of a third generation pertussis vaccine for humans.
Similar content being viewed by others
INTRODUCTION
Whooping cough is a respiratory infectious disease caused by the Gram-negative bacterium Bordetella pertussis, which can be lethal in unimmunized infants. Studies in mice have shown that IFN-γ, produced by Th1 cells and NK cells, plays a critical role in protection against primary infection with B. pertussis and in adaptive immunity against re-infection.1,2,3 Mice defective in the IFN-γ receptor suffer from disseminated infection and often die from the disease.3 Natural infection with B. pertussis also induces Th17 cells, and there is emerging evidence from mouse and baboon models that Th17 cells also play a role in protective immunity against this bacterium.4,5
Whole cell pertussis (wP) vaccines induce potent Th1 and Th17 responses in animal models4 and are effective at preventing pertussis disease in vaccinated children.6 Adverse effects associated with wP vaccines resulted in their replacement with safer acellular pertussis (aP) vaccines,6,7,8 which were introduced into routine immunization programs in many developed countries in the late 1990s. However, in recent years, the incidence of whooping cough has increased in a number of countries with high vaccination coverage, including the United States, the Netherlands, England, Australia, and Ireland.9,10,11,12 There are a number of explanations for the resurgence of pertussis, including better diagnosis, the emergence of antigen variants and waning or suboptimal immunity induced with aP vaccines.13 The current aP vaccines, which are formulated with alum as the adjuvant, induce potent antibody and Th2-polarized responses in humans and animal models.4,14 While aP vaccines can also induce weak Th17 responses, they are inefficient at promoting Th1 cells.4 Furthermore, when compared with natural infection or immunization with wP vaccines, current aP vaccines appear to be less effective at inducing immunological memory and in conferring long-term protection against pertussis.15,16
We have recently demonstrated that lung CD4 tissue-resident memory (TRM) cells play a critical role in adaptive immunity induced by previous infection with B. pertussis.17 It has also been reported that mucosal immunization with BCG is more effective than parenteral immunization for induction of lung CD4 TRM cells.18 Therefore, the waning immunity observed following immunization with current parenterally-delivered alum-adjuvanted aP vaccines16 may reflect a failure to induce TRM cells in the respiratory tract. We and others have reported that replacing alum with Th1-promoting adjuvants, including the Toll-like receptor (TLR) agonists, CpG (TLR9) or LP1569 (TLR2), can enhance the protective efficacy of experimental aP vaccines in mice.4,19,20 It has also been reported that intranasal (i.n.) administration of c-di-GMP 24 h prior to challenge with B. pertussis significantly reduced the bacterial load in the lungs.21 C-di-GMP is an agonist for the intracellular receptor stimulator of interferon genes (STING), a cytosolic pattern recognition receptor that senses DNA, resulting in activation of innate immune cells.22 Furthermore, c-di-GMP can act as an adjuvant and can synergize with TLR agonists to enhance immune responses to nasally delivered antigens.23,24,25,26
We have previously demonstrated that a TLR2 agonist from B. pertussis, LP1569, is an effective adjuvant for an experimental aP vaccine.20 In this study, we examine the hypothesis that an adjuvant combination of TLR2 and STING agonists may be capable of promoting sustained Th1 and Th17 responses and superior long-term protection against B. pertussis infection than an aP vaccine formulated with alum, and that i.n. immunization may be a better immunization route for induction of B. pertussis-specific memory T cells. Our findings reveal that i.n. immunization of mice with an experimental aP vaccine formulated with a combination of LP1569 and c-di-GMP, termed LP-GMP, induced significant numbers of respiratory IL-17-secreting CD4 TRM cells and prevented nasal colonization for at least 10 months after immunization.
RESULTS
C-di-GMP and LP1569 synergistically induce Th1/Th17 polarizing cytokines and maturation of dendritic cells
Th1 and Th17 cells play a critical role in protective immunity to B. pertussis.4 In this study, we sought to identify an adjuvant combination that promoted production of Th1 and Th17 polarizing cytokines and maturation of dendritic cells (DCs). Most TLR agonists induce the Th1 polarizing cytokine IL-12, but are more variable at inducing the Th17-polarizing cytokines IL-23 and IL-1β. We hypothesized that the Th17 response induced with a TLR agonist could be augmented by addition of another pathogen-associated molecular pattern (PAMP). In preliminary experiments, we found that the STING agonist c-di-GMP augmented IL-1β and IL-23 as well as IL-12 production by DCs, induced by the TLR9, TLR2 or TLR7/8 agonists CpG, R848 and LP1569 respectively (data not shown). LP1569 was chosen over CpG or R848 for further study because it was a derivative of BP1569, a natural adjuvant and antigen from B. pertussis.20
We examined the immunomodulatory activity of a combination of the TLR2 agonist LP1569 and the STING agonist c-di-GMP. Stimulation of murine bone marrow-derived DCs with c-di-GMP alone induced IFN-β production, but little or no IL-12p70, IL-1β or IL-23. LP1569 alone induced low concentrations of IL-12p40, IL-12p70 and IL-1β, but no IL-23 or IFN-β. In contrast, the combination of LP1569 and c-di-GMP (termed LP-GMP) induced significant concentrations of IL-12p40, IL-12p70, IL-1β, IL-23 and IFN-β, which were greater than those induced by either LP1569 or c-di-GMP alone (Fig. 1a). DCs stimulated with c-di-GMP, but not LP1569, also induced IL-10, which was significantly enhanced by the addition of LP1569 (Fig. 1a). The combination of c-di-GMP and LP1569 also markedly enhanced the expression of CD80, CD86, CD40 and MHC class II on DCs (Fig. 1b).
TLR2 and STING agonists synergistically enhance Th1 and Th17 polarizing cytokines and maturation of murine and human DCs. a, b Murine bone marrow-derived DCs were stimulated with c-di-GMP (GMP; 10 μg/ml), LP1569 (LP; 1 μg/ml) or a combination of the two agonists (LP-GMP). a Production of Th1 and Th17 polarizing cytokines, IL-10 and type I IFN was quantified by ELISA after 24 h. Results are the mean ± SEM of triplicate stimulations and are representative of three independent experiments. ***P < 0.001 vs. all other groups except where specifically indicated, by one-way ANOVA with the Bonferroni post-test. b Expression of CD80, CD86, CD40 and MHC class II was assessed by flow cytometry after 24 h. The data are representative FACS plots from three independent experiments. c–e Human DCs were stimulated with 2’3’-cGAMP (cGAMP; 10 μg/ml), LP1569 (LP; 10 μg/ml) or a combination of the two agonists (LP-cGAMP). c Production of Th1 and Th17 polarizing cytokines was quantified by ELISA after 24 h. Results are the mean ± SEM of triplicate stimulations. **P < 0.01, ***P < 0.001 vs. cGAMP; ++P < 0.01, +++P < 0.001 vs. LP by one-way ANOVA with the Bonferroni post-test. d Human PBMCs were stimulated with anti-CD3 and anti-CD28 in the presence of medium (Med) only, 2’3’-cGAMP (10 µg/ml), LP1569 (10 µg/ml) or both. After 72 h, cells surface stained with anti-CD3 and anti-CD4 and stained intracellularly with anti-IL-17 and anti-IFN-γ. Flow cytometric analysis was performed. Results are mean (±SEM) frequency of IL-17+ or IFN-γ+ CD3+CD4+ T cells for triplicate stimulations. e Expression of CD40, CD80 and MHC class II (HLA-DR) was assessed by flow cytometry after 24 h. The data are representative FACS plots from three independent experiments
We next assessed if the combination of these TLR2 and STING agonists could activate human immune cells in vitro. Stimulation of human DCs with 2′3′-cGAMP, an agonist for human STING, significantly augmented LP1569-induced IL-6, IL-12p70 and IL-23p19 and also non-significantly enhanced IL-1β production (Fig. 1c). We indirectly assessed the capacity of 2’3’-cGAMP, LP1569 or the combination to promote human Th1 and Th17 responses in vitro. Human peripheral blood mononuclear cells (PBMCs) were stimulated for 72 h and flow cytometry was performed after intracellular cytokine staining for IL-17 and IFN-γ and surface staining for CD4. The results revealed that the combination of 2′3′-cGAMP and LP1569 induced higher concentrations of IL-17 and significantly higher concentrations of IFN-γ than either 2’3’-cGAMP or LP1569 alone (Fig. 1d). Finally, we assessed the capacity of 2’3’-cGAMP, LP1569 or the combination to induce human DC maturation. We found that the combination of 2′3′-cGAMP and LP1569 induced expression of CD40, CD80 and HLA-DR on human DCs, which was greater than that induced by 2′3′-cGAMP or LP1569 alone (Fig. 1e). These findings demonstrate that the combination of TLR2 and STING agonists synergistically enhance Th1 and Th17 polarizing cytokines, and maturation of mouse and human DCs.
Since our ultimate aim was to use LP-GMP as an adjuvant for a nasal vaccine, we evaluated potential toxicity by assessing its ability to promote inflammatory cytokines in the olfactory bulb and brain of mice. Neuronal effects have been reported following i.n. delivery of nasal GM1 monosialoganglioside binding toxins, cholera toxin (CT) and E. coli heat-labile enterotoxin (LT).27,28,29 CT delivered to mice by the i.n. route induced IL-1β mRNA expression in the olfactory bulb and hypothalamus, but LP-GMP did not enhance IL-1β or TNF mRNA expression in the olfactory bulb or IL-1β mRNA in the hypothalamus (Supplementary Figure 1). These findings suggest that i.n. administration of LP-GMP is not associated with induction of inflammatory cytokines in the olfactory bulb or brain, previously reported for GM1-binding toxins.27
A TLR2-STING agonist combination promotes antigen-specific Th1 and Th17 cell responses to a model antigen in vivo
We assessed the adjuvant activity of the LP1569 and c-di-GMP combination using the model antigen KLH. Potent antigen-specific Th1 and Th17 responses were detected in draining lymph nodes from mice after a single immunization with KLH and LP-GMP (Fig. 2). Furthermore, the background IL-5 observed in other experimental groups was significantly reduced in mice immunized with KLH and LP-GMP. Immunization with KLH and LP1569 alone induced modest IFN-γ production, whereas KLH with c-di-GMP alone induced potent IFN-γ production but weaker IL-17 production than observed with LP-GMP. These findings indicate that the adjuvant combination LP-GMP induces robust Th1 and Th17 cell responses against a model antigen in vivo.
A TLR2-STING agonist combination is an effective adjuvant and promotes antigen-specific Th1 and Th17 responses to a model antigen in vivo. Mice were injected in the footpad with PBS, KLH (1 μg/mouse), KLH + c-di-GMP (KLH + GMP; 10 μg/mouse), KLH + LP1569 (KLH + LP; 50 μg/mouse) or KLH + LP-GMP. Seven days later, the popliteal lymph nodes were isolated and cells were restimulated with increasing concentrations of KLH (2, 50, 100 μg/ml). After 72 h, KLH-specific cytokine production in the supernatants was assessed by ELISA. Results are representative of two experiments and mean ± SEM values for 3 mice per group. **P < 0.01, ***P < 0.001 vs. PBS; ++P < 0.01, +++P < 0.001 vs. KLH; ◆◆P < 0.01, ◆◆◆P < 0.001 vs. KLH + GMP; #P < 0.05, ##P < 0.01, ###P < 0.001 vs. KLH + LP by two-way ANOVA with the Bonferroni post-test
An experimental aP vaccine adjuvanted with LP-GMP induces robust antigen-specific IFN-γ and IgG2c production and protection against B. pertussis infection
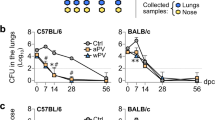
We examined the ability of LP-GMP to act as an adjuvant for an experimental 2-component aP vaccine (filamentous hemagglutinin; FHA and recombinant pertussis toxin; rPT) in an established respiratory infection model.30 Current aP vaccines used in humans have 1–5 antigens and those with more antigens are most effective.30,31 We used 2 antigens at a low dose (0.2 µg/mouse) to determine if our adjuvant combination was effective with a ‘weak’ vaccine. Mice were immunized with the experimental aP vaccine (rPT and FHA) twice (0 and 4 weeks) and challenged by aerosol infection with B. pertussis 2 weeks after the second immunization (Supplementary Figure 2a). C-di-GMP alone was a relatively poor adjuvant for the experimental aP vaccine, with only a 2 log10 reduction in CFU counts post challenge. LP1569 was a better adjuvant, but the best protection was observed in mice immunized with an experimental aP vaccine formulated with the LP-GMP combination (Fig. 3a). An examination of the areas under the bacterial clearance curves, which gives an overall measure of protection, revealed that the bacterial load was lowest in mice immunized with the aP vaccine and LP-GMP (26.7), when compared with aP and LP1569 (43.4), aP and c-di-GMP (62.8) or PBS (79.5). Furthermore, the CFU counts were significantly lower after B. pertussis challenge of mice immunized with the aP vaccine and LP-GMP compared with the same vaccine formulated with alum (Fig. 3b).
An aP vaccine formulated with LP-GMP induces potent Th1 and Th17 cell responses and confers greater protection against B. pertussis infection, than an experimental alum-adjuvanted aP vaccine. Mice were immunized i.p. twice (0 and 4 weeks) with PBS or a 2-component aP vaccine comprising FHA and rPT (0.2 μg/mouse) formulated with either c-di-GMP (10 μg/mouse), LP1569 (50 μg/mouse) or LP-GMP (50 μg LP1569 + 10 μg c-di-GMP / mouse). Immunized mice were challenged by exposure to live B. pertussis 2 weeks after the last immunization. a Lung CFU counts (mean ± SEM n = 4 mice per group per time-point). *P < 0.05, **P < 0.01, ***P < 0.001 vs. aP + c-di-GMP; +P < 0.05 vs. aP + LP1569 by two-way ANOVA with the Bonferroni post-test. b Mice were immunized i.p. twice (0 and 4 weeks) with PBS or a 2-component aP vaccine formulated with LP-GMP (50 μg LP1569 + 10 μg c-di-GMP / mouse) or alum (100 μg/mouse). Lung CFU counts (mean ± SEM n = 4 mice per group per time-point). *P < 0.05, ***P < 0.001 vs. PBS; #P < 0.05, ##P < 0.01, ###P < 0.001 vs. aP + alum by two-way ANOVA with the Bonferroni post-test. c Two weeks after the last immunization, spleen cells (2 × 106 cells/ml) were cultured with FHA (0.5 or 2 μg/ml), sonicated B. pertussis (Son Bp; 1 or 10 μg/ml) or medium (Med) and after 72 h IFN-γ, IL-17 and IL-5 concentrations in supernatants were quantified by ELISA. Results shown in each panel are mean ± SEM values (n = 4 mice). *P < 0.05, **P < 0.01, ***P < 0.001 vs. PBS; #P < 0.05, ##P < 0.01, ###P < 0.001 vs. aP + alum by two-way ANOVA with the Bonferroni post-test. d FHA-specific antibody titers in sera were quantified by ELISA (mean ± SEM values; n = 4 mice). ***P < 0.001 vs. PBS; ###P < 0.001 vs. aP + alum by one-way ANOVA with the Bonferroni post-test. (e) 2 weeks after the last immunization, mice were injected with an anti-CD45 antibody i.v. 10 min prior to killing. Lungs were isolated and cells were stained with antibodies specific for CD4, CD3, CD8, CD44, CD62L, CD69 and CD103 for FACS analysis. Results are expressed as absolute numbers of CD45-CD69+ TRM (CD45−CD4+CD8−CD44+CD62L−CD69+), IL-17+ TRM cells (CD45−CD4+CD8−CD44+CD62L−CD69+) and IFN-γ+ TRM cells (CD45-CD4+CD8−CD44+CD62L−CD69+) in the lungs. *P < 0.05, **P < 0.01 vs. PBS; #P < 0.05 vs. aP + alum by one-way ANOVA with the Bonferroni post-test. Results are representative of 3 experiments
Assessment of cellular immune responses in immunized mice prior to aerosol challenge revealed that spleen cells from mice immunized with the aP vaccine formulated with LP-GMP produced significantly more IFN-γ and IL-17 in response to FHA or sonicated B. pertussis than those from mice immunized with the aP vaccine containing alum (Fig. 3c). In contrast, antigen-specific IL-5, but not IFN-γ or IL-17, was produced by spleen cells from mice immunized with the aP vaccine formulated with alum.
Assessment of serum antibody responses revealed robust FHA-specific IgG1 titers in the sera of mice immunized with the experimental aP vaccine formulated with either LP-GMP or with alum. However, mice immunized with the aP vaccine and LP-GMP had significantly more serum FHA-specific IgG2c antibodies compared with mice immunized with the experimental aP vaccine containing alum (Fig. 3d).
Assessment of memory T cells in the lungs on the day of challenge revealed that immunization with aP and LP-GMP induced modest numbers of CD4+CD69+ TRM and IFN-γ or IL-17-secreting TRM cells in the lungs, which were substantially greater than those induced with aP and alum, which were close to background levels (Fig. 3e). Collectively, our findings demonstrate that the novel adjuvant combination promotes induction of Th1, Th17 and opsonizing IgG2c antibodies, and this is reflected by substantially better protection against lung infection with B. pertussis than that induced with the alum-adjuvanted aP vaccine, which induces IgG1 and Th2 responses. The aP vaccine with LP-GMP also promotes TRM cell migration to the lungs, but not as effectively as we had previously reported in mice infected with B. pertussis.17
Intranasal immunization with an aP vaccine and LP-GMP induces potent TRM cells
Since mucosal infection or immunization is more effective than parenteral immunization for inducing TRM cells in the lungs,18 we assessed the i.n. route of immunization. For human use, aP vaccines can include 1–5 antigens. In the studies on parenteral immunization described above, we used an aP vaccine with 2 antigens (rPT and FHA) because it allowed us to clearly discriminate between good and very good vaccine-adjuvant combinations. However, in a preliminary experiment, we found that i.n. immunization with an experimental 2-component aP vaccine lacking pertactin (PRN) was not very effective at preventing infection (Fig. 4a). In contrast, mice immunized i.n. twice with a 3-component aP vaccine (rPT, FHA and PRN) formulated with LP-GMP cleared the B. pertussis infection from the lungs as rapidly as mice immunized i.p. with the same experimental vaccine (Fig. 4b). Bacteria were at very low levels in the lungs 7 days after challenge in mice immunized with the 3-component aP vaccine with the novel adjuvant combination by either route.
Intranasal immunization with a 3-component aP vaccine formulated with LP-GMP induces potent TRM cells and confers protection against lung infection with B. pertussis. a Mice were immunized i.n. twice (0 and 4 weeks) with an experimental 2-component aP vaccine (FHA and rPT; 0.2 μg/mouse) formulated with LP-GMP (50 μg LP1569 + 10 μg c-di-GMP/mouse). Control mice were immunized with PBS. Immunized mice were challenged by exposure to live B. pertussis 2 weeks after the last immunization. The results show lung CFU counts (mean ± SEM n = 4 mice per group per time-point. b Mice were immunized i.n. twice (0 and 4 weeks) with an experimental 3-component aP vaccine (FHA, rPT and PRN; 0.2 μg/mouse) formulated with LP-GMP (as above) or with PBS. Immunized mice were challenged by exposure to live B. pertussis 2 weeks after the last immunization. The results show lung CFU counts (mean ± SEM n = 4 mice per group per time-point). c Prior to challenge, (2 weeks after the last immunization), spleen cells (2 × 106 cells/ml) were cultured with PRN (2 μg/ml), FHA (0.5 or 2 μg/ml), Son Bp (1 or 10 μg/ml) or medium as a negative control and after 72 h IFN-γ and IL-17 concentrations in supernatants were quantified by ELISA. Results are mean ± SEM values (n = 4 mice). **P < 0.01, ***P < 0.001 vs. PBS; ++P < 0.01, +++P < 0.001 vs. aP + LP-GMP (i.p.); ◆◆◆P < 0.001 vs. aP + LP-GMP (i.n.) by two-way ANOVA with the Bonferroni post-test. d, e Prior to challenge, lung mononuclear cell preparations were labeled with antibodies specific for CD4, CD3, CD8, CD44, CD62L, CD69 and CD103 for FACS analysis. Results are shown as (d) sample FACS plots for CD69 versus CD103 on CD4+CD8−CD44+CD62L−CD69+ cells and (e) mean ± SEM (n = 4 mice) absolute numbers of CD69+ TRM (CD4+CD8−CD44+CD62L−CD69+) and CD69+CD103+ TRM cells (CD4+CD8-CD44+CD62L-CD69+). **P < 0.01 vs. PBS; ++P < 0.01 vs. aP + alum by one-way ANOVA with the Bonferroni post-test. Results are representative of 2 experiments
Immunization with the aP vaccine and LP-GMP by the i.n. route generated potent antigen-specific IL-17 production, especially against PRN (Fig. 4c). In contrast, i.p. immunization with the same experimental vaccine generated predominantly Th1-type responses; high concentrations of IFN-γ were produced by spleen cells in response to FHA, PRN and B. pertussis sonicate (Fig. 4c).
Assessment of memory T cells in the lungs on the day of challenge revealed that i.n. immunization with aP and LP-GMP was highly effective in the induction of CD4+CD69+ or CD4+CD69+CD103+ TRM cells (Fig. 4d, e). Although the same vaccine delivered parenterally also promoted TRM cells in the lungs, there were significantly more CD4 TRM cells and IL-17-secreting TRM cells in the lungs of mice immunized by the i.n. route. These findings demonstrate that the i.n. route is an effective means of generating Th17 cells and memory T cells in the lungs, and in conferring protective immunity against lung infection with B. pertussis.
Intranasal immunization with an aP vaccine formulated with LP-GMP protects against nasal colonization with B. pertussis
Studies in baboons have suggested that, although immunization with an alum-adjuvanted aP vaccine prevents lung infection, it fails to prevent nasal colonization and transmission of B. pertussis.5 We hypothesized that nasal immunization with our novel adjuvant combination that promotes Th1 and Th17 cells may be more effective than parenteral immunization with an alum-adjuvanted aP vaccine for preventing B. pertussis colonization of the nasal cavity, as well as infection of the lungs. We found that immunization with an experimental aP vaccine formulated with LP-GMP delivered by the i.p. or i.n. route offered a high level of protection against lung infection with B. pertussis (Fig. 5a). When compared with non-immunized mice, the CFU counts in the lungs after B. pertussis aerosol challenge were also significantly reduced in mice immunized with the alum-adjuvanted aP vaccine, but the reduction was not as rapid as that seen in mice immunized with aP and LP-GMP.
Intranasal immunization with an aP vaccine formulated with LP-GMP protects against nasal colonization with B. pertussis. Mice were immunized i.n. twice (0 and 4 weeks) with an experimental 3-component aP vaccine (FHA, rPT and PRN, 0.2 μg/mouse) formulated with LP-GMP (as described in Fig. 4). Control mice were immunized with PBS. Immunized mice were challenged by exposure to live B. pertussis 2 weeks after the last immunization. CFU counts (mean ± SEM; n = 4 mice per group per time-point) in the lungs (a) and nose (b). *P < 0.05, ***P < 0.001 vs. aP + alum by two-way ANOVA with the Bonferroni post-test. c Prior to challenge, (2 weeks after the last immunization), spleen cells (2 × 106 cells/ml) were cultured with FHA (2 μg/ml), sonicated B. pertussis (10 μg/ml) or medium as a negative control and after 72 h IFN-γ and IL-17 concentrations in supernatants were quantified by ELISA. Results are mean ± SEM values (n = 4 mice). d, e On the day of but prior to challenge, (2 weeks after the last immunization), mice were injected with an anti-CD45 antibody i.v. 10 min prior to killing. Lungs were isolated and cells were stained with antibodies specific for CD4, CD3, CD8, CD44, CD62L, CD69 and CD103 for FACS analysis. d Sample FACS plots of CD69 vs. CD45i.v. (labeled in vivo; negative cells are lung resident) and CD69 versus CD103 gated on CD45−, CD4+ CD3+, CD8−, CD44+, CD62L− cells. e Absolute numbers of CD45−CD69+ TRM (CD45−CD4+CD8−CD44+CD62L−CD69+), IL-17+CD45−CD4+ T cells in the lungs. **P < 0.01 vs. PBS; ###P < 0.001 vs. aP + alum, +++P < 0.001 vs. aP + LP-GMP (i.p.) by one-way ANOVA with the Bonferroni post-test. Results are representative of 3 experiments
Parenteral immunization of mice with the aP vaccine formulated with alum had a very limited ability to reduce nasal colonization after B. pertussis aerosol challenge; the CFU counts in the nose were still at or above those in non-immunized control mice 14 days post challenge (Fig. 5b). In contrast, parenteral immunization with the experimental aP vaccine formulated with LP-GMP significantly reduced the CFU counts in the nose by 14 days post challenge. However, the most dramatic effect on nasal colonization was observed with the aP vaccine formulated with LP-GMP and delivered by the i.n. route (Fig. 5b). The CFU counts in the nose were already close to the detection limits of the assay 3 days after aerosol challenge with B. pertussis. These findings demonstrate that an aP vaccine with the novel adjuvant combination LP-GMP when delivered by the i.n. route induces sterilizing immunity against B. pertussis in the lungs and the nose.
Assessment of antigen-specific immune responses in the spleen on the day of challenge revealed that i.p. immunization with the experimental aP vaccine formulated with the LP-GMP adjuvant combination induced predominantly Th1 responses by the parenteral route and Th17 dominated responses by the nasal route (Fig. 5c). These results also further highlighted the limited ability of a low-dose alum-adjuvanted aP vaccine to induce Th1 and Th17-type responses.
Assessment of the TRM cells in the lungs on the day of challenge revealed that immunization with the experimental aP vaccine formulated with LP-GMP by the i.n. route was a highly effective approach for inducing respiratory TRM cells (Fig. 5d, e). A substantial proportion of these were IL-17-secreting lung tissue-resident CD4+ cells (Fig. 5e). CD69+ or CD69+CD103+ TRM were also detected in the lungs, albeit at much lower levels, in mice immunized i.p. with aP formulated with LP-GMP. In contrast, TRM cells were not expanded in the lung prior to infection with B. pertussis in mice immunized with the alum-adjuvanted aP vaccine. These findings demonstrated that while the alum-adjuvanted aP vaccine fails to induce Th1, Th17 or respiratory TRM cells, potent cellular immune responses, including memory T cells, are induced by immunization of mice with an aP vaccine formulated with the novel adjuvant combination LP-GMP. Furthermore, immunization by the i.n. route was an efficient method for inducing B. pertussis-specific Th17 cells and respiratory TRM cells, and for conferring protection against nasal colonization as well as lung infection with B. pertussis.
Our initial adjuvant combination was selected on the basis of protection against lung infection following parenteral immunization. Therefore, we assessed the relative ability of LP1569, c-di-GMP or the combination LP-GMP to induce memory T cells and to confer protection against nasal colonization as well as lung infection with an experimental aP vaccine delivered by the i.n. route. Immunization with the 3-component aP vaccine formulated with either LP1569, c-di-GMP or LP-GMP all conferred significant protection against lung infection, but the most complete protection was observed with LP-GMP as the adjuvant (Fig. 6a). Furthermore, i.n. immunization with aP formulated with LP-GMP was highly effective in preventing nasal colonization and reduced the bacterial load in the nose to background levels by day 6 (Fig. 6b). Nasal immunization with aP formulated with either LP1569 or c-di-GMP also promoted protection against nasal colonization, but the reduction in bacterial counts in the nose was not as consistent across the 3 time points when compared with LP-GMP (Fig. 6b). Assessment of memory T cells in the lung 6 days post challenge revealed that LP1569, c-di-GMP and LP-GMP each promoted the induction of CD69+ TRM cells (Fig. 6c, d). However, addition of c-di-GMP significantly enhanced the number of CD69+ TRM cells and IL-17-secreting CD69+ TRM cells induced with LP1569. Although the numbers of IFN-γ-secreting TRM cells were lower than IL-17-secreting TRM cells, aP formulated with LP-GMP induced significantly more IFN-γ-secreting CD69+ TRM cells than the aP vaccine formulated with LP1569 or c-di-GMP (Fig. 6c, d). These findings demonstrate that while LP1569 and c-di-GMP are effective adjuvants for nasal immunization with an aP vaccine, the LP-GMP adjuvant combination may be more potent for promoting sustained protective immunity.
LP-GMP is a potent adjuvant combination for an aP vaccine delivered by the i.n. route. Mice were immunized i.n. twice (0 and 4 weeks) with an experimental 3-component aP vaccine (FHA, rPT and PRN, 0.2 μg/mouse) formulated with LP-GMP (as described in Fig. 4), c-di-GMP only, LP1569 only or with PBS. Immunized mice were challenged by exposure to live B. pertussis 2 weeks after the last immunization. CFU counts (mean ± SEM; n = 4 mice per group per time-point) in the lungs (a) and nose (b). *P < 0.05, ***P < 0.001 versus aP + c-di-GMP; ###P < 0.001 vs. aP + LP-GMP by two-way ANOVA with the Bonferroni post-test. On day 6 post-challenge, mice were injected with an anti-CD45 antibody i.v. 10 min prior to euthanasia. Lungs were isolated and cell subsets were determined by flow cytometry as described in Fig. 5. Representative FACS plots of CD69 versus CD45i.v. (top row), CD69 vs. IFN-γ (middle row) and CD69 vs. IL-17 (bottom row) gated on CD45−, CD3+, CD4+, CD44+, CD62L− cells (c). Absolute numbers of CD69+ TRM, IFN-γ+CD69+ TRM cells and IL-17+CD69+ TRM cells in the lungs (d). *P < 0.05, **P < 0.01 by one-way ANOVA with the Bonferroni post-test
Immunization with an aP vaccine formulated with LP-GMP confers long-term protection against lung infection and nasal colonization with B. pertussis
Protective immunity against pertussis disease in children wanes rapidly even after a course of up to 5 immunizations with aP vaccines formulated with alum.16 We hypothesized that a vaccine that induced potent cellular immune responses and memory T cells, especially those that are resident in respiratory tissue, was more likely to maintain protective immunity against infection in the lungs and nose. Therefore, we examined the ability of the novel aP vaccine to sustain protective immunity against B. pertussis. Mice were immunized twice i.p or i.n. (4 weeks apart) with the 3-component aP vaccine and LP-GMP, or i.p. with aP and alum, and challenged with B. pertussis after 10 months (Supplementary Figure 2b). Mice immunized i.p. or i.n. with aP and LP-GMP had very low CFU counts in the lungs on days 3 and 7 post challenge, and bacteria were completely cleared by day 14 (Fig. 7a). Immunization with the aP vaccine formulated with alum also sustained protection in the lungs, though not as effectively as that seen with aP formulated with LP-GMP.
Immunization with an aP vaccine formulated with LP-GMP confers long-term protection against lung infection and nasal colonization with B. pertussis. Mice were immunized i.n. or i.p. twice (0 and 4 weeks) with an experimental 3-component aP vaccine (FHA, rPT and PRN; 0.2 μg/mouse) formulated with either LP-GMP or with alum (as described in Fig. 3). Control mice were immunized with PBS. Immunized mice were challenged by exposure to live B. pertussis 10 months after the last immunization. Results show CFU counts (mean ± SEM; n = 4 mice per group per time-point) in the lungs (a) and nose (b). *P < 0.05, **P < 0.01, ***P < 0.001 vs. PBS; #P < 0.05 vs. aP + alum by two-way ANOVA with the Bonferroni post-test. c Prior to challenge, (10 months after the last immunization), spleen cells (2 × 106 cells/ml) were cultured with FHA (2 μg/ml), PRN (2 μg/ml) and sonicated B. pertussis (10 μg/ml) or medium and after 72 h IFN-γ and IL-17 concentrations in supernatants were quantified by ELISA. Results are mean ± SEM values (n = 4 mice per groups). **P < 0.01, ***P < 0.001 vs. PBS; #P < 0.05, ##P < 0.01 vs. aP + alum; ++P < 0.01 vs. aP + LP-GMP (i.p.) by two-way ANOVA with the Bonferroni post-test. d PRN-specific antibody titers in sera on the day of challenge were quantified by ELISA. *P < 0.05, **P < 0.01 vs. PBS by one-way ANOVA with the Bonferroni post-test. e PRN-specific IgA titers in the lungs 3 days post challenge were quantified by ELISA. *P < 0.05, **P < 0.01 vs. PBS; ##P < 0.01 vs. aP + alum by one-way ANOVA with the Bonferroni post-test. f Flow cytometry analysis was performed on mononuclear cells prepared from nasal tissue recovered 14 days post challenge. Absolute numbers of CD69+ TRM cells, IL-17+CD69+ TRM cells and IFN-γ+CD69+ TRM cells were determined by flow cytometry as described in Fig. 5. **P < 0.01 vs. PBS; #P < 0.05, ##P < 0.01 vs. aP + alum by one-way ANOVA with the Bonferroni post-test. Results in d-f are from a single experiment with 4 mice per group; horizontal bar = mean values
The aP vaccine formulated with LP-GMP delivered by the i.n. route was the most effective vaccine at conferring long-term protection against nasal colonization (Fig. 7b). Protective immunity in the nose induced with the same vaccine delivered parenterally also persisted for at least 10 months. However, the aP vaccine with alum failed to generate long protective immunity in the nose.
Assessment of the persistence of the immune response following vaccination revealed that strong antigen-specific Th1 and weaker Th17 responses persisted in the spleen for at least 10 months after parenteral immunization with aP and LP-GMP (Fig. 7c). Furthermore, strong Th17 and weaker Th1 responses persisted in mice immunized i.n. with aP and LP-GMP. Consistent with our earlier data, antigen-specific IL-17 and IFN-γ were very low or undetectable in mice immunized with aP and alum. Furthermore, PRN-specific IgG, especially IgG2c, was strongest in mice immunized with aP and LP-GMP delivered by the i.p or i.n routes (Fig. 7d). Finally, PRN-specific IgA in the lungs was at background level 3 days after challenge in mice immunized with aP and alum, but significant levels of PRN-specific IgA were detected in the lungs of mice immunized with aP and LP-GMP, especially by the i.n. route (Fig. 7e).
We next examined the persistence of the cellular immune response in the nasal tissue, including memory T cells, after immunization with the different experimental aP vaccines. Using mononuclear cells from nasal tissue prepared by mechanical and enzymatic disruption, we found that CD4+CD69+ TRM cells were expanded post B. pertussis challenge in mice immunized i.n. with aP and LP-GMP (Fig. 7f). Furthermore, a significant number of these nasal tissue-resident cells secreted IL-17 and a smaller proportion secreted IFN-γ. IL-17 or IFN-γ secreting CD4+CD69+ TRM were also detected in the nasal cavity post B. pertussis challenge of mice immunized i.p with aP and LP-GMP (Fig. 7f). In contrast, the numbers of CD69+ TRM cells, IL-17-secreting CD4+CD69+ TRM and IFN-γ-secreting CD4+CD69+ TRM in the nasal cavities of mice immunized with aP and alum were at or below those seen in non-immunized mice.
Finally, using a previously validated approach for quantifying protection in mice,30 we found a significant correlation between the rate of bacterial clearance from the nose and the number of IL-17-secreting CD4+CD69+ TRM cells in the nasal tissue (Fig. 8). Collectively these findings demonstrate that LP-GMP is a more effective adjuvant than alum for inducing Th1, Th17 and TRM cells, and reveal that nasal immunization with aP and LP-GMP is a highly effective approach for inducing protective IL-17-secreting CD4 TRM cells in the nasal tissue.
Long-term protection against nasal colonization correlates with the numbers of IL-17+ TRM cells in the nasal tissue. Mice were immunized and challenged 10 months later with B. pertussis as described in Fig. 6. The area under the curves for bacterial colonization of the nose on days 0 to 14 after challenge, expressed as a ratio of area under the curves for control mice immunized with PBS, was plotted against the number of IL-17+ TRM cells in the nasal tissue as described in Fig. 7
DISCUSSION
In this study, we have developed an approach for generating long-term protective immunity against nasal colonization of mice with B. pertussis, which involved mucosal immunization with a non-replicating vaccine that induced high numbers of IL-17-secreting respiratory TRM cells. We identified a novel adjuvant combination, LP-GMP, comprising an activator of the intracellular bacterial DNA sensor STING and a ligand for TLR2, which synergistically activated mouse and human DCs and promoted potent Th1 and Th17 responses in vivo. When combined with protective antigens from B. pertussis, the adjuvant combination promoted induction of protective immunity against nasal colonization as well as lung infection, and when delivered by the i.n. route was particularly effective at inducing respiratory IL-17-secreting TRM cells that sustained protective immunity against nasal colonization.
Pertussis is a re-emerging disease in many countries with high vaccine coverage and this may reflect asymptomatic transmission of B. pertussis from individuals immunized with the current aP vaccine.13,32 It may also reflect waning immunity due to a failure to induce immunological memory, especially TRM cells. Recent evidence from a baboon model has suggested that although commercial alum-adjuvanted aP vaccines are capable of preventing severe disease, they do not prevent B. pertussis colonization of the respiratory tract or transmission to unvaccinated baboons.5 Our data in the mouse model are consistent with those from the baboon model and provide further evidence of the limitations of aP vaccines formulated with alum. While immunization with an alum-adjuvanted aP vaccine was moderately effective at preventing infection of the lungs, it did not prevent colonization of the nose with B. pertussis. In contrast, an aP vaccine formulated with LP-GMP, a novel adjuvant formulation that promoted Th1, Th17 and respiratory TRM cells conferred immunity against nasal colonization as well as lung infection.
Although much of the research on the mechanisms of protective immunity has focused on antibody responses, there is now convincing evidence that T cells play a crucial role in natural and optimal vaccine-induced immunity to B. pertussis.14 Studies in humans and in mice have demonstrated that infection with B. pertussis or immunization with wP vaccines induce potent antigen-specific Th1 responses,33,34,35 which are required for clearance of B. pertussis from the respiratory tract.4 Studies in IFN-γR−/− mice have demonstrated that this cytokine is crucial for clearing a primary infection with B. pertussis from the lungs and for mediating adaptive immunity induced by previous infection or immunization with a wP vaccine.3,4 IFN-γ produced by Th1 cells and NK cells promotes production of opsonizing antibodies and activates macrophages to kill intracellular bacteria in the respiratory tract.36,37 Consistent with previous studies that have substituted alum with TLR agonists as the adjuvant in experimental aP vaccines,4,19,20 we found that LP-GMP promoted potent Th1 responses with an experimental aP vaccine, especially when delivered parenterally.
IL-17 and Th17 cells also play a critical role in protective immunity to B. pertussis by helping to recruit neutrophils to the site of infection4 and promoting induction of antimicrobial peptides,38 which can kill B. pertussis.39 We found that in addition to promoting Th1 responses, the combination adjuvant LP-GMP was highly effective at inducing Th17 cells. This reflected the ability of the STING agonist to augment TLR2-induced IL-1β and IL-23 production by murine and human DCs, cytokines that activate and expand Th17 cells.38 Interestingly, the most potent memory Th17 responses were observed when the LP-GMP formulated aP vaccine was delivered by the i.n. route and this correlated with optimum protection against nasal colonization. This is consistent with a report that parenteral delivery of a tuberculosis subunit vaccine protected against aerosolised M. tuberculosis infection by inducing robust Th1 immunity, whereas i.n. delivery of the same vaccine switched the immune profile from Th1 to Th17 in the lungs and spleen, without affecting the protective efficacy of the vaccine.40
Intranasal delivery of vaccines with AB-type bacterial toxins as adjuvants has been associated with adverse events. A study in Switzerland reported that humans immunized nasally with an inactivated influenza virosome vaccine containing enzymatically active Escherichia coli heat-labile toxin (LT) as the adjuvant had a higher risk of developing Bell’s palsy.41 Furthermore, two cases of transient Bell’s palsy were reported in human volunteers in phase 1 clinical trials of the nasal subunit HIV and tuberculosis vaccines that included enzymatically inactive non-toxic mutant LT (LTK63) as the adjuvant.29 Studies in mice have shown that i.n. delivery of the related bacterial toxin, CT, can undergo retrograde transport to olfactory bulbs of the central nervous system through binding to GM1.28 Furthermore, i.n. administered CT can induce proinflammatory responses in the olfactory bulbs and brain.27 However, we found that, unlike the GM1-binding adjuvant, CT, our novel adjuvant combination LP-GMP did not induce IL-1β or TNF in the olfactory bulb or brain, suggesting that it will not be associated with neuronal toxicity.
Vaccines delivered by the mucosal route have the advantage of inducing local secretory IgA. Indeed, a live attenuated B. pertussis vaccine BPZE1 delivered by the i.n. route was shown to induce local IgA as well as systemic Th1 responses, and conferred protection against B. pertussis infection in mice.42 We found that i.n. immunization with an aP formulated with LP-GMP induced weak FHA-specific IgA (unpublished data) and modest PRN-specific IgA responses in the respiratory tract. While we do not rule out a role for IgA, our data points to a role for cellular immune responses in combination with opsonizing antibodies. We found that an aP vaccine formulated with LP-GMP induced robust PRN-specific IgG responses, and interestingly the ratio of IgG2c/IgG1 was significantly higher in mice immunized with aP and LP-GMP by parenteral or mucosal routes when compared with aP and alum delivered parenterally. Furthermore, PRN appeared to be an important antigen for Th17 responses and its inclusion with PT and FHA in a nasally delivered experimental aP vaccine significantly augmented protection. PRN has been a target antigen for immune-driven antigenic variation and its deletion in certain circulating strains of B. pertussis underlines its importance in protective immunity to this pathogen.43 Our data suggest that PRN may play an important role in generating host immunity against B. pertussis colonization of the nasal tract. Notwithstanding the fact that PRN-negative B. pertussis strains are emerging, it would seem prudent to keep PRN in the aP vaccine. It might also be useful to identify antigens that have not been the subject of immune-driven pressure as additional components of a next generation pertussis vaccine.
Immunity induced in children with current alum-adjuvanted aP vaccines wanes rapidly after immunization, even after 5 doses of the vaccine.16 Recent studies have highlighted the importance of local memory T cells in the tissues, termed TRM cells. There is convincing evidence that CD8 TRM cells mediate immunity to viral infection in mucosal tissues.44 It has also been reported that CD4 TRM cells mediate adaptive immunity to influenza infection in the lungs45 and there is emerging evidence of a role for CD4 TRM cells in immunity to bacterial infections.46 We have previously reported that CD4 TRM cells play an important role in immunity generated by previous infection with B. pertussis.17 The current study provides evidence that CD4 TRM cells may be critical for long-term vaccine-induced immunity to B. pertussis. The emerging view is that TRM cells do not migrate to tissues following parenteral immunization, whereas infection or immunization at mucosal sites is effective at inducing memory T-cell recruitment to the tissues. It has been reported that significantly more IL-17+ CD4+ effector memory T cells are recruited to the lungs of mice primed s.c. and boosted i.n. with a vaccine containing a Th1/Th17-promoting adjuvant.47 It has also been demonstrated that i.n. immunization with BCG induced the infiltration of greater numbers of CD4+ and CD8+ TRM cells to the lungs than s.c. vaccination.18 We found a significant number of CD4+CD69+ TRM cells in the lungs on the day of challenge in mice immunized i.n. with aP and LP-GMP, but not in mice immunized i.p. with aP formulated with alum. Furthermore, CD4+CD69+ TRM cells were expanded in the nasal tissue after challenge in mice immunized with aP and LP-GMP especially by the i.n. route, but not in mice immunized with aP and alum. Moreover, the CD4+CD69+ TRM cells induced in the lungs and nose by i.n. immunization with aP and LP-GMP were predominantly IL-17-secreting. Finally, protection against nasal colonization significantly correlated with the number of IL-17-secreting CD4+CD69+ TRM cells in nasal tissue.
Our findings suggest that i.n immunization with an aP vaccine formulated with TLR2 and STING agonists is an effective approach for inducing IL-17-secreting TRM cells and for conferring long-term protection against nasal colonization with B. pertussis. We also provide proof-of-principle that the TLR2 and STING agonists combination can promote maturation and production of Th1/Th17 polarizing cytokines by human as well as mouse DCs and could therefore form the basis of a third generation pertussis vaccine for humans.
METHODS
Mice
C57BL/6 mice were purchased from Harlan, UK or were bred in house in the Comparative Medicine Unit in Trinity College Dublin. Mice were 6–10 weeks old at the initiation of experiments and were housed under specific pathogen-free conditions. Mice were maintained according to the regulations of the European Union and Irish Department of Health and Children. Experiments were performed under licence from the Irish Health Products Regulatory Authority and with approval from the Trinity College Dublin Ethics committee.
Mouse immunization
For footpad immunizations, mice were immunized once s.c. in the footpad with KLH48 (1 μg/mouse), KLH + c-di-GMP (10 μg/mouse), KLH + LP1569 (50 μg/mouse) or KLH + LP-GMP or PBS. Alternatively, mice were immunized twice at 0 and 4 weeks with experimental aP vaccines, consisting of 2 or 3 B. pertussis antigens, FHA (Kaketsuken, Kumamoto, Japan), recombinant pertussis toxin (rPT; LIST Laboratories; #184) with or without PRN (LIST Laboratories; #187) formulated with LP156920 and/or c-di-GMP (Invivogen), or aluminium hydroxide (Alhydrogel; Brenntag Biosector, Denmark). For i.p. immunizations, mice were immunised with 200 μl of the experimental aP vaccine into the peritoneal cavity. For i.n. immunizations, mice were lightly anaesthetized with isoflurane to minimize their stress during immunization. The experimental vaccine (20 µl) was administered by placing two 10 µl droplets on the mouse nares which were subsequently inhaled during the natural breathing process.
B. pertussis respiratory challenge
Respiratory infection of mice was performed 2 weeks or 10 months after the second immunization by aerosol challenge with a culture of B. pertussis (1 × 109 CFU/ml) as described.17 The course of B. pertussis infection was followed by performing CFU counts on the lungs or nose of mice/group/time-point. The lungs or nose were aseptically removed and homogenized in 1 ml (lungs) or 500 µl (nose) of 1% casein solution. Undiluted and serially diluted homogenates (100 μl) from individual lungs or noses were spotted in duplicate onto Bordet-Gengou agar plates and the number of CFU was calculated after 5−6 days incubation at 37 °C.
T-cell cytokine production
For experiments involving i.p. or i.n. immunization with experimental vaccines, the spleens were isolated 2 weeks or 10 months after the second i.p. or i.n. immunization. For the experiment involving s.c. immunization with KLH into the footpad, the popliteal lymph nodes were isolated 1 week after immunization. Cells were cultured at a concentration of 2 × 106/ml (spleen) or 1 × 106/ml (lymph node), at 37 °C and 5% CO2 with the antigens KLH (2, 50 or 100 μg/ml), FHA (0.5 or 2 μg/ml), PRN (2 μg/ml) or sonicated B. pertussis (1 or 10 μg/ml). Supernatants were removed after 72 h and IFN-γ, IL-17 and IL-5 concentrations were determined by two-site ELISA.
Antibody production
Sera were obtained from immunized mice by cardiac puncture and serum antibody responses to B. pertussis were quantified by ELISA using the B. pertussis antigens FHA or PRN (1 μg/ml). Bound antibodies were detected using biotin-conjugated anti-mouse IgG (Sigma-Aldrich) or IgG1 (BD Pharmingen), or HRP-conjugated IgG2c (Bio-Rad Laboratories). PRN-specific IgA in lung homogenates was quantified by ELISA using an antibody specific for mouse IgA (Southern Biotech). Antibody levels are expressed as the mean endpoint titer (±SE), determined by extrapolation of the linear part of the titration curve to 2 SE above the background value obtained with non-immune mouse serum.
Dendritic cell culture
DCs were expanded from bone marrow from naive C57BL/6 mice using GM-CSF as described.49 Human DCs were generated from PBMCs of healthy donors following a protocol modified from Baleeiro et al.50 Briefly, PBMC derived monocytes were cultured in RPMI with GM-CSF (50 ng/ml) and IL-4 (40 ng/ml) (both Peprotech) for 7 days.
Human in vitro T-cell responses
PBMCs from healthy donors were stimulated with plate-bound anti-CD3 (2 µg/ml; eBioscience) and soluble anti-CD28 (2 µg/ml; Invitrogen) in the presence of 2′3′-cGAMP (10 µg/ml), LP1569 (10 µg/ml) or both. After 72 h, cells were washed and incubated with LIVE/DEAD fixable Aqua (Invitrogen), before being surface stained in the presence of Fcγ block (Human TruStain FcX; BioLegend) with anti-CD3-APC-H7 (BD Pharmingen) and anti-CD4-PerCP-ef710 (eBioscience). Cells were fixed in Fix/Perm buffer, permeabilized with Perm buffer (eBioscience), and stained intracellularly with anti-IL-17-APC and anti-IFN-γ-PE-Cy7. Flow cytometric analysis was performed on an LSR Fortessa, and data were acquired using the Diva software (BD Biosciences). The results were analyzed using FlowJo (TreeStar, V10).
Cytokine expression in the olfactory bulbs and hypothalamus
CT (Sigma; 10 µg/mouse), or the combination of LP1569 (50 µg/mouse) and c-di-GMP (10 µg/mouse) were administered i.n. to mice in 10 µl PBS without anaesthesia. Control mice received PBS. Mice were euthanized 6 h post treatment and the olfactory bulbs and the hypothalamus were dissected, pressed through a 70 µm cell strainer and centrifuged. The pellets were resuspended in TRIzol (Ambion) and stored at –80 °C. mRNA was prepared according to the manufacturer’s protocol and transcribed into cDNA (MultiScribe Reverse Transcriptase; Invitrogen) for analysis. IL-1β and TNF mRNA expression were determined via RT-PCR (TaqMan Assay Mm00434228_m1 and Mm00443258_m1), using 18 S rRNA as the housekeeping gene (Euk 18 S rRNA; Applied Biosystems).
Detection of murine respiratory tissue-resident cells
We employed an established technique to distinguish tissue-resident from circulating CD4 T cells.17 Mice were injected with an anti-CD45 antibody (BioLegend) intravenously (i.v.) 10 min prior to euthanasia. Circulating lymphocytes are exposed to the antibody and are labeled CD45+, whereas tissue-resident cells are ‘protected’ and remain CD45−. Lung and nasal tissue mononuclear cell suspensions were prepared by mechanical (chopping with a scalpel) followed by enzymatic disruption of tissue for 1 h with Collagenase D (1 mg/ml; Sigma-Aldrich) and DNAse I (20 U/ml; Sigma-Aldrich).
Flow Cytometry Analysis
Bone marrow-derived DCs were stained with antibodies specific for CD80 (16-10A1; BioLegend), CD86 (GL-1; BD Pharmingen), CD40 (3/23; BD Pharmingen), MHC class II (M5/114.15.2; eBioscience), and Fcγ block (1 µg/ml; eBioscience). Human DCs were stained with antibodies specific for CD40 (C53; eBioscience) and HLA-DR (AC122; Miltenyi), and Fcγ block (Biolegend). Cells were washed and fixed with 50 μl 2% PFA (Thermo Scientific) for 15 min at room temperature and analyzed by flow cytometry.
Single cell suspensions from lungs or nasal tissue were incubated in FACS tubes (1 × 106 cells/tube) and stained with Live/Dead aqua stain for 20 min and then washed with PBS. Cells were incubated with the antibodies specific for CD3 (17A2; BioLegend), CD4 (RM4-5; eBioscience), CD8 (53-6.7; eBioscience), CD44 (IM7; BioLegend), CD62L (MEL-14; BD Biosciences), CD69 (H1.2F3; eBioscience) and CD103 (M290; BD Biosciences) in the presence of Fcγ block for 20 min on ice in the dark. After fixing, cells were analyzed by flow cytometry.
For intracellular cytokine staining, cells were stimulated for 4 h with PMA (5 ng/ml; Sigma-Aldrich), ionomycin (500 ng/ml; Sigma-Aldrich) and brefeldin A (5 μg/ml; Sigma-Aldrich). Cells were fixed in 2% PFA and permeabilized with 0.5% saponin (Sigma-Aldrich, Ireland), followed by staining with antibodies specific for IFN-γ (XMG1.2; eBioscience) or IL-17 (eBio17B7; eBioscience). Fluorescence minus one or nonspecific isotype antibodies were used as controls. Flow cytometric analysis was performed on an LSR Fortessa, and data were acquired using Diva software (BD Biosciences). The results were analyzed using FlowJo software (TreeStar).
Statistical analysis
Statistical analysis was carried out using GraphPad Prism 5. One or two-way analysis of variance (ANOVA) followed by the Bonferroni post-test was used to analyze the statistical significance between three or more groups. P values less than 0.05 were considered to be statistically significant.
References
Barbic, J., Leef, M. F., Burns, D. L. & Shahin, R. D. Role of gamma interferon in natural clearance of Bordetella pertussis infection. Infect. Immun. 65, 4904–4908 (1997).
Sakurai, S. et al. Nitric oxide induction by pertussis toxin in mouse spleen cells via gamma interferon. Infect. Immun. 64, 1309–1313 (1996).
Mahon, B. P., Sheahan, B. J., Griffin, F., Murphy, G. & Mills, K. H. Atypical disease after Bordetella pertussis respiratory infection of mice with targeted disruptions of interferon-gamma receptor or immunoglobulin mu chain genes. J. Exp. Med 186, 1843–1851 (1997).
Ross, P. J. et al. Relative contribution of Th1 and Th17 cells in adaptive immunity to Bordetella pertussis: towards the rational design of an improved acellular pertussis vaccine. PLoS Pathog. 9, e1003264 (2013).
Warfel, J. M., Zimmerman, L. I. & Merkel, T. J. Acellular pertussis vaccines protect against disease but fail to prevent infection and transmission in a nonhuman primate model. Proc. Natl Acad. Sci. USA 111, 787–792 (2014).
Simondon, F. et al. A randomized double-blind trial comparing a two-component acellular to a whole-cell pertussis vaccine in Senegal. Vaccine 15, 1606–1612 (1997).
Greco, D. et al. A controlled trial of two acellular vaccines and one whole-cell vaccine against pertussis. Progetto Pertosse Working Group. N. Engl. J. Med 334, 341–348 (1996).
Gustafsson, L., Hallander, H. O., Olin, P., Reizenstein, E. & Storsaeter, J. A controlled trial of a two-component acellular, a five-component acellular, and a whole-cell pertussis vaccine. N. Engl. J. Med 334, 349–355 (1996).
de Melker, H. E. et al. Pertussis in The Netherlands: an outbreak despite high levels of immunization with whole-cell vaccine. Emerg. Infect. Dis. 3, 175–178 (1997).
Amirthalingam G., Gupta S., Campbell H. Pertussis immunisation and control in England and Wales, 1957 to 2012: a historical review. Euro Surveill. 18, 20587 (2013).
Pillsbury, A., Quinn, H. E. & McIntyre, P. B. Australian vaccine preventable disease epidemiological review series: pertussis, 2006-2012. Commun. Dis. Intell. Q. Rep. 38, E179–E194 (2014).
Barret A. S. et al. Pertussis outbreak in northwest Ireland, January - June 2010. Euro Surveill. 15, 198654 (2010).
Mills, K. H., Ross, P. J., Allen, A. C. & Wilk, M. M. Do we need a new vaccine to control the re-emergence of pertussis? Trends Microbiol. 22, 49–52 (2014).
Higgs, R., Higgins, S. C., Ross, P. J. & Mills, K. H. Immunity to the respiratory pathogen Bordetella pertussis. Mucosal Immunol. 5, 485–500 (2012).
Klein, N. P., Bartlett, J., Fireman, B., Rowhani-Rahbar, A. & Baxter, R. Comparative effectiveness of acellular versus whole-cell pertussis vaccines in teenagers. Pediatrics 131, e1716–e1722 (2013).
Klein, N. P., Bartlett, J., Rowhani-Rahbar, A., Fireman, B. & Baxter, R. Waning protection after fifth dose of acellular pertussis vaccine in children. N. Engl. J. Med. 367, 1012–1019 (2012).
Wilk, M. M. et al. Lung CD4 Tissue-Resident Memory T Cells Mediate Adaptive Immunity Induced by Previous Infection of Mice with Bordetella pertussis. J. Immunol. 199, 233–243 (2017).
Perdomo C. et al. Mucosal BCG vaccination induces protective lung-resident memory T cell populations against tuberculosis. mBio 7, e01686-16 (2016).
Asokanathan C., Corbel M., Xing D. A CpG-containing oligodeoxynucleotide adjuvant for acellular pertussis vaccine improves the protective response against Bordetella pertussis. Hum. Vaccin. Immunother. 9, 325–331 (2013).
Dunne, A. et al. A novel TLR2 agonist from Bordetella pertussis is a potent adjuvant that promotes protective immunity with an acellular pertussis vaccine. Mucosal Immunol. 8, 607–617 (2015).
Elahi, S. et al. c-di-GMP enhances protective innate immunity in a murine model of pertussis. PloS ONE 9, e109778 (2014).
Yin, Q. et al. Cyclic di-GMP sensing via the innate immune signaling protein STING. Mol. Cell 46, 735–745 (2012).
Yan, H. et al. 3’,5’-Cyclic diguanylic acid elicits mucosal immunity against bacterial infection. Biochem. Biophys. Res. Commun. 387, 581–584 (2009).
Ebensen, T., Schulze, K., Riese, P., Morr, M. & Guzman, C. A. The bacterial second messenger cdiGMP exhibits promising activity as a mucosal adjuvant. Clin. Vaccin. Immunol. 14, 952–958 (2007).
Temizoz B., et al. TLR9 and STING agonists synergistically induce innate and adaptive type-II IFN. Eur. J. Immunol. 45, 1159–1169 (2014).
Yildiz, S. et al. Enhanced immunostimulatory activity of cyclic dinucleotides on mouse cells when complexed with a cell-penetrating peptide or combined with CpG. Eur. J. Immunol. 45, 1170–1179 (2015).
Armstrong, M. E., Lavelle, E. C., Loscher, C. E., Lynch, M. A. & Mills, K. H. Proinflammatory responses in the murine brain after intranasal delivery of cholera toxin: implications for the use of AB toxins as adjuvants in intranasal vaccines. J. Infect. Dis. 192, 1628–1633 (2005).
van Ginkel, F. W., Jackson, R. J., Yuki, Y. & McGhee, J. R. Cutting edge: the mucosal adjuvant cholera toxin redirects vaccine proteins into olfactory tissues. J. Immunol. 165, 4778–4782 (2000).
Lewis, D. J. et al. Transient facial nerve paralysis (Bell’s palsy) following intranasal delivery of a genetically detoxified mutant of Escherichia coli heat labile toxin. PLoS ONE 4, e6999 (2009).
Mills, K. H., Ryan, M., Ryan, E. & Mahon, B. P. A murine model in which protection correlates with pertussis vaccine efficacy in children reveals complementary roles for humoral and cell-mediated immunity in protection against Bordetella pertussis. Infect. Immun. 66, 594–602 (1998).
Zhang L., Prietsch S. O., Axelsson I., Halperin S. A. Acellular vaccines for preventing whooping cough in children. Cochrane Database Syst. Rev. Cd001478 (2012).
Althouse, B. M. & Scarpino, S. V. Asymptomatic transmission and the resurgence of Bordetella pertussis. BMC Med. 13, 146 (2015).
Ryan, M. et al. Bordetella pertussis respiratory infection in children is associated with preferential activation of type 1 T helper cells. J. Infect. Dis. 175, 1246–1250 (1997).
Ryan, M. et al. Distinct T-cell subtypes induced with whole cell and acellular pertussis vaccines in children. Immunology 93, 1–10 (1998).
Mills, K. H., Barnard, A., Watkins, J. & Redhead, K. Cell-mediated immunity to Bordetella pertussis: role of Th1 cells in bacterial clearance in a murine respiratory infection model. Infect. Immun. 61, 399–410 (1993).
Byrne, P., McGuirk, P., Todryk, S. & Mills, K. H. Depletion of NK cells results in disseminating lethal infection with Bordetella pertussis associated with a reduction of antigen-specific Th1 and enhancement of Th2, but not Tr1 cells. Eur. J. Immunol. 34, 2579–2588 (2004).
Mahon, B. P. & Mills, K. H. Interferon-gamma mediated immune effector mechanisms against Bordetella pertussis. Immunol. Lett. 68, 213–217 (1999).
Mills, K. H. Induction, function and regulation of IL−17-producing T cells. Eur. J. Immunol. 38, 2636–2649 (2008).
Fernandez, R. C. & Weiss, A. A. Susceptibilities of Bordetella pertussis strains to antimicrobial peptides. Antimicrob. Agents Chemother. 40, 1041–1043 (1996).
Orr, M. T. et al. Mucosal delivery switches the response to an adjuvanted tuberculosis vaccine from systemic TH1 to tissue-resident TH17 responses without impacting the protective efficacy. Vaccine 33, 6570–6578 (2015).
Mutsch, M. et al. Use of the inactivated intranasal influenza vaccine and the risk of Bell’s palsy in Switzerland. N. Engl. J. Med 350, 896–903 (2004).
Feunou, P. F., Kammoun, H., Debrie, A. S., Mielcarek, N. & Locht, C. Long-term immunity against pertussis induced by a single nasal administration of live attenuated B. pertussis BPZE1. Vaccine 28, 7047–7053 (2010).
Martin, S. W. et al. Pertactin-negative Bordetella pertussis strains: evidence for a possible selective advantage. Clin. Infect. Dis. 60, 223–227 (2015).
Turner, D. L. et al. Lung niches for the generation and maintenance of tissue-resident memory T cells. Mucosal Immunol. 7, 501–510 (2014).
Teijaro, J. R. et al. Cutting edge: Tissue-retentive lung memory CD4 T cells mediate optimal protection to respiratory virus infection. J. Immunol. 187, 5510–5514 (2011).
Sakai, S. et al. Cutting edge: control of Mycobacterium tuberculosis infection by a subset of lung parenchyma-homing CD4 T cells. J. Immunol. 192, 2965–2969 (2014).
Christensen D., Mortensen R., Rosenkrands I., Dietrich J., Andersen P. Vaccine-induced Th17 cells are established as resident memory cells in the lung and promote local IgA responses. Mucosal Immunol. 10, 260–270 (2016).
Lavelle, E. C. et al. Cholera toxin promotes the induction of regulatory T cells specific for bystander antigens by modulating dendritic cell activation. J. Immunol. 171, 2384–2392 (2003).
Sutton, C. E. et al. Loss of the molecular clock in myeloid cells exacerbates T cell-mediated CNS autoimmune disease. Nat. Commun. 8, 1923 (2017).
Baleeiro, R. B. et al. Direct activation of human dendritic cells by particle-bound but not soluble MHC class II ligand. PLoS ONE 8, e63039 (2013).
Acknowledgements
This work was supported by grants from Science Foundation Ireland awarded to Kingston Mills (11/PI/1036, 16/IA/4468 and 12/RI/2340)
Author information
Authors and Affiliations
Contributions
K.H.G.M. planned the project, designed the experiments, analyzed and interpreted the data. A.C.A., M.M.W., A.M. and L.B. designed and performed experiments and analyzed the data. D.M. performed the experiments on human DCs. A.C.A. and K.H.G.M. wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
K.H.G.M. is an inventor on a patent application filed around LP1569. The other declare no competing interests.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Allen, A.C., Wilk, M.M., Misiak, A. et al. Sustained protective immunity against Bordetella pertussis nasal colonization by intranasal immunization with a vaccine-adjuvant combination that induces IL-17-secreting TRM cells. Mucosal Immunol 11, 1763–1776 (2018). https://doi.org/10.1038/s41385-018-0080-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41385-018-0080-x
This article is cited by
-
IL-17 and IL-17-producing cells in protection versus pathology
Nature Reviews Immunology (2023)
-
Intranasal COVID-19 vaccine induces respiratory memory T cells and protects K18-hACE mice against SARS-CoV-2 infection
npj Vaccines (2023)
-
CD4+ T cell memory
Nature Immunology (2023)
-
How immunology can help reverse the pertussis vaccine failure
Nature Immunology (2023)
-
Tissue adaptation and clonal segregation of human memory T cells in barrier sites
Nature Immunology (2023)