Abstract
Although macrophages (Mϕ) maintain intestinal immune homoeostasis, there is not much available information about their subset composition, phenotype and function in the human setting. Human intestinal Mϕ (CD45+HLA-DR+CD14+CD64+) can be divided into subsets based on the expression of CD11c, CCR2 and CX3CR1. Monocyte-like cells can be identified as CD11chighCCR2+CX3CR1+ cells, a phenotype also shared by circulating CD14+ monocytes. On the contrary, their Mϕ-like tissue-resident counterparts display a CD11c−CCR2−CX3CR1− phenotype. CD11chigh monocyte-like cells produced IL-1β, both in resting conditions and after LPS stimulation, while CD11c− Mϕ-like cells produced IL-10. CD11chigh pro-inflammatory monocyte-like cells, but not the others, were increased in the inflamed colon from patients with inflammatory bowel disease (IBD), including Crohn's disease and ulcerative colitis. Tolerogenic IL-10-producing CD11c− Mϕ-like cells were generated from monocytes following mucosal conditioning. Finally, the colonic mucosa recruited circulating CD14+ monocytes in a CCR2-dependent manner, being such capacity expanded in IBD. Mϕ subsets represent, therefore, transition stages from newly arrived pro-inflammatory monocyte-like cells (CD11chighCCR2+CX3CR1+) into tolerogenic tissue-resident (CD11c−CCR2−CX3CR1−) Mϕ-like cells as reflected by the mucosal capacity to recruit circulating monocytes and induce CD11c− Mϕ. The process is nevertheless dysregulated in IBD, where there is an increased migration and accumulation of pro-inflammatory CD11chigh monocyte-like cells.
Similar content being viewed by others
INTRODUCTION
Inflammatory bowel disease (IBD), a chronic inflammatory disorder of the gastrointestinal (GI) tract, can be divided into two entities, named Crohn's disease (CD) and ulcerative colitis (UC). CD and UC differ according to their distribution, type of inflammation, symptoms and associated complications.1,2 Both diseases are thought to be the consequence of an exacerbated immune response towards the commensal microbiota3 and display an increase in both their incidence and prevalence in Western societies.4 Unfortunately, there is currently no curative treatment for CD or UC, so the goal is to induce clinical remission which, however, is only achieved in a proportion of the patients.5 Therefore, a deeper insight is needed into the mechanisms controlling IBD pathogenesis aiming to identify the dysregulated immune pathways leading to tissue inflammation in those patients.
Macrophages (Mϕ) are professional antigen-presenting cells highly adapted to the tissue that they inhabit.6 In the intestine, Mϕ are highly specialized to avoid overt immunity in response to the gut microbiota.7,8,9 Most current knowledge about Mϕ biology has been obtained from murine models, which have revealed that GI-Mϕ, as opposed to those from other tissues which are typically derived from yolk sac or foetal liver precursors, are constantly replenished from circulating Ly6Chigh monocytes entering the GI-mucosa in a CCR2-dependent manner.10,11 Newly arrived Ly6Chigh monocytes are subsequently conditioned by the tissue microenvironment through several intermediates via the “monocyte waterfall”,9,10,11,12,13 which results in the differentiation of tissue-resident tolerogenic Mϕ (Ly6C−MHC-IIhighCX3CR1highCCR2−). Mature GI-Mϕ maintain local homoeostasis via their hyporesponsiveness to microbial stimulation, but also via removal of apoptotic/dead cells and microbes, mediation of tissue and epithelial remodelling and secretion of regulatory cytokines, including IL-10, which maintains the survival of local regulatory T-cells (Tregs). However, this process is disrupted during intestinal inflammation where there is enhanced recruitment of monocytes and their differentiation towards tissue tolerogenic Mϕ is abrogated.8,912,14,15,16,17
In humans, Mϕ are expanded in the GI-mucosa in active IBD patients, where they also display an immature phenotype characterized by lower HLA-DR expression compared with the healthy tissue.10,18,19,20,21,22,23 However, it is currently unknown whether the same migration and differentiation processes described in the murine models also apply in the human context, not just in the steady state but also under inflammatory conditions including IBD. Therefore, here we have characterized GI-Mϕ subset composition, phenotype and function in healthy controls. Indeed, we describe how mature intestinal tolerogenic Mϕ can be identified as CD11−CCR2−CX3CR1− cells, as opposed to pro-inflammatory monocyte-like cells which are expanded in the inflamed mucosa from IBD patients and display a CD11highCCR2+CX3CR1+ phenotype.
RESULTS
CD11c expression identifies different Mϕ subsets throughout the healthy human GI tract
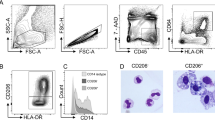
Human intestinal Mϕ were identified within singlet viable leucocytes (CD45+) as CD14+CD64+HLA-DR+ (Figs. 1a, b). Given that the properties of the immune system change systematically through the GI tract,24 we first determined whether Mϕ numbers varied through its length, revealing that Mϕ numbers were higher in the large bowel (either proximal or distal colon) compared with the small bowel (terminal ileum or duodenum) (Fig. 1c).
Human intestinal macrophages. a Human intestinal macrophages (Mϕ) were identified within singlet viable leucocytes as CD14+CD64+ (red gating) by flow cytometry on total lamina propria mononuclear cells (LPMC). b Further analysis confirmed that all Mϕ were HLA-DR+ as opposed to the CD14−CD64− fraction (grey histogram) which was predominantly HLA-DR−. c Total Mϕ numbers (referred to the total number of viable LPMC) were higher in the human colon (both proximal and distal) compared with the terminal ileum and the duodenum. d Human intestinal Mϕ were divided into CD11chigh (black), CD11cdim (red) and CD11c− (blue) subsets as referred to the Fluorescence Minus One (FMO) control, e each of them displaying different levels of autofluorescence. f Mϕ subsets changed their relative proportions through the human gastrointestinal tract, with the CD11chigh subset being predominant in the colon as opposed to CD11c− Mϕ which were higher in the duodenum. For c, f, samples from the distal colon, proximal colon and terminal ileum were obtained from the same controls (when access to the ileum was available) while duodenal samples were obtained from independent donors. Results from c, f also denote samples from the same individuals, considered either a total Mϕ (c) or divided into subsets (f). One-way ANOVA repeated measures and subsequent Tukey correction (c, f) were applied to compare Mϕ numbers between the distal colon, proximal colon and terminal ileum while duodenal samples were compared with the other three by t-test. p-Values < 0.05 were considered significant (*p < 0.05)
Further analysis revealed that the CD11c integrin displayed different levels of expression on human GI-Mϕ, which allowed us to discriminate three different subsets of CD11chigh, CD11cdim and CD11c− Mϕ (Fig. 1d and Supplementary Figure 1). Indeed, those subsets displayed differences in their autofluorescence, which was higher on the CD11c− subset (Fig. 1e). Moreover, Mϕ relative proportion changed throughout the GI tract as the CD11chigh subset was predominant in distal compartments (proximal colon and distal colon) as opposed to their CD11c− counterparts, which were expanded in the small bowel (Fig. 1f).
CD11chigh Mϕ are CCR2+CX3CR1+ and display a “monocyte-like” phenotype
We next characterized the phenotype of human GI-Mϕ on the basis of their CD11c expression. Typically, CD11cdim Mϕ displayed an intermediate or transition phenotype between the CD11chigh and the CD11c− subsets, with the latter displaying higher levels of CD64 and HLA-DR, together with lower expression of SIRPα (Fig. 2a). Although the presence of differences in the expression of CD14 has been previously suggested, which may resemble the murine Ly6C waterfall,10,12 in our hands CD14 was not differentially expressed in the different CD11c subsets (Fig. 2a).
Characterization of human intestinal macrophage subsets. a Human colonic macrophage (Mϕ) subsets were identified as in Fig. 1d and characterized for the expression of CD14, CD64, HLA-DR and SIRPα. Shaded histograms denote the expression for each marker on the CD14−CD64− fraction. b Mϕ subsets were further characterized for the expression of Mϕ-associated markers including CCR2, CD40 and CX3CR1; c as well as CD86, CD206 and CD163. Given the differences in the autofluorescence displayed by the different Mϕ subsets (Fig. 1e), the percentage of positive cells for each marker on each given Mϕ subset was determined based on their specific Fluorescence Minus One (FMO). One-way ANOVA repeated measures with Tukey correction was applied in all cases. p-Values < 0.05 were considered significant (*p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001)
Provided that Mϕ subsets displayed differences in their autofluorescence (Fig. 1e), we next applied specific Fluorescence Minus One (FMOs) in order to determine the percentage of positive cells for each marker on each specific subset. The expression of monocyte-associated markers like CCR2 or CD40, as well as the Mϕ-associated marker CX3CR1, were restricted to the CD11chigh subset (Fig. 2b). However, activation marker CD86 and scavenger receptors CD206 and CD163 were not influenced by the CD11c expression waterfall (Fig. 2c).
Having characterized human GI-Mϕ on the basis of CD11c, we next assessed the expression of the markers differentially expressed between GI-Mϕ subsets on their circulating precursors. Hence, human circulating monocytes were identified and divided into classical (CD14+CD16−), intermediate (CD14+CD16+) and non-classical (CD14−CD16+) monocytes (Fig. 3a). While CX3CR1 was expressed by all subsets with virtually no differences between them, intermediate monocytes displayed higher levels of both HLA-DR and CD40 compared with their classical and non-classical counterparts (Figs. 3b, c). CCR2 expression, nevertheless, was specifically associated with CD14+ monocytes (either classical or intermediate) as it was absent on non-classical monocytes (Figs. 3b, c). Hence, intestinal CD11chigh Mϕ display a monocyte-like phenotype as both intestinal CD11chigh Mϕ and circulating CD14+ monocytes are CCR2+CX3CR1+, although the expression of these monocyte-associated markers were reduced on the CD11cdim subset and absent on CD11c− Mϕ (Fig. 2b).
Human circulating monocyte subsets. a Total circulating monocytes were identified within peripheral blood mononuclear cells from healthy controls and divided into classical (CD14+CD16−), intermediate (CD14+CD16+) and non-classical (CD14−CD16+). b Monocyte subsets were further characterized for the expression of HLA-DR, CX3CR1, CD40 and CCR2. Shaded histograms denote the expression for each marker on the HLA-DR− fraction. Pooled data from several independent experiments are shown in c. d Human colonic macrophages (Mϕ) were divided into subsets based on the expression of CD11c (CD11chigh, CD11cdim, CD11clow), CCR2 (CCR2+, CCR2−) and CX3CR1 (CX3CR1+, CX3CR1−). Further analysis of the expression for these markers was determined within each specific subset. Results are representative from five independent experiments performed with similar results. One-way ANOVA repeated measures with Tukey correction was applied in c. p-Values <0.05 were considered significant (*p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001)
Intestinal Mϕ were next divided into subsets based on the expression of CCR2 and CX3CR1, which as opposed to CD11c (which shows a continuum on its expression) display a bimodal distribution (Fig. 3d). When Mϕ are divided into subsets based on these markers, a clear correlation between the expression of CD11c, CCR2 and CX3CR1 is found (Fig. 3d). Together, our results suggest that monocyte-like cells can be identified in the mucosa as CD11chighCCR2+CX3CR1+, a phenotype also shared by circulating CD14+ monocytes, while tissue-resident Mϕ display a CD11c−CCR2−CX3CR1− phenotype. Therefore, and in order to get a deeper insight into the characterization of monocyte-like and Mϕ-like cells, cells were further divided into subsets based on the intensity of CD11c, although similar results should be obtained in the cells that are divided based on the expression of CCR2 or CX3CR1.
Monocyte-like cells preferentially produce IL-1β as opposed to their Mϕ-like counterparts which secrete IL-10
Having described differences in the phenotype of human GI-Mϕ, we next determined whether this was also coupled with a differential function. Monocyte-like cells (identified as CD11chigh) displayed a pro-inflammatory biased profile with high spontaneous production of IL-1β. Mϕ-like cells (identified as CD11c−), on the contrary, spontaneously produced larger amounts of IL-10, while intermediate cells (identified as CD11cdim) displayed a transition phenotype between both subsets. TNFα production, however, was minimal and was not associated with any subset (Fig. 4a). Similar results were obtained if the Mϕ were divided into subsets based on the expression of CCR2 and CX3CR1 (Supplementary Figure 2), hence confirming that monocyte-like CD11chighCCR2+CX3CR1+ cells preferentially produce IL-1β as opposed to their CD11c−CCR2−CX3CR1− Mϕ-like counterparts which display an enhanced production of IL-10.
CD11chigh macrophages produce larger amounts of IL-1β while CD11c− macrophages secrete IL-10, both in resting conditions and after LPS stimulation. a Human colonic macrophage (Mϕ) subsets were identified as in Fig. 1d and their spontaneous production of intracellular IL-1β, TNFα and IL-10 determined. Positive and negative gatings for each cytokine on each subset were determined by comparison with their respective Fluorescence Minus One (FMO). b Intracellular production of IL-1β and c IL-10 was further determined for each Mϕ subset following 18 h culture of LPMC in the presence/absence of lipopolysaccharide (LPS). One-way ANOVA repeated measures with Tukey correction was applied in a, while two-way ANOVA repeated measures with Sidak correction was applied in b, c. p-Values <0.05 were considered significant (*p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001)
We next assessed whether these functional differences were reflected in their responsiveness to bacterial stimulation using TLR4 ligand lipopolysaccharide (LPS). CD11chigh and CD11cdim Mϕ produced IL-1β in response to LPS (Fig. 4b), whereas CD11c− Mϕ enhanced their production of IL-10 following LPS challenge (Fig. 4c). Together, these cytokine profiles indicate a more inflammatory profile for CD11chigh cells, fitting with their monocytic profile, and a tolerogenic profile for CD11c− cells in keeping with intestinal Mϕ-like properties.
Higher numbers of CD11chigh monocyte-like pro-inflammatory cells in the inflamed mucosa from IBD patients
Having described phenotypical and functional differences between pro-inflammatory CD11high monocyte-like cells, transition CD11dim cells and tolerogenic CD11− Mϕ-like cells, we next studied their relative abundance in the IBD mucosa. Given that total Mϕ numbers (Fig. 1c), as well as their relative proportion (Fig. 1f), change through the human GI tract, here we specifically focused on the human colon, hence abrogating the regional effect. Total Mϕ were present in higher numbers in the inflamed colon from IBD patients (Fig. 5a), as previously described.18,19,20,21,22,23 Indeed, colonic Mϕ numbers were higher in the inflamed mucosa from both active UC and CD patients, but not in the non-inflamed colon from the same patients or in quiescent ones (Fig. 5b). When Mϕ subset composition was considered (Fig. 5c), it became evident that it was specifically the pro-inflammatory CD11chigh monocyte-like subset, but not the others, the population present in higher numbers in the inflamed mucosa from IBD patients (Fig. 5d).
CD11chigh macrophages are expanded in the inflamed colon from IBD patients. a Total macrophages (Mϕ), determined within colonic lamina propria mononuclear cells as in Fig. 1a, were expanded in the inflamed colon from a patient with active ulcerative colitis (aUC) but not on the non-inflamed mucosa from the same patient. b Pooled data from several independent experiments displaying total Mϕ on the colonic mucosa from healthy controls, as well as IBD patients including the inflamed and non-inflamed mucosa from aUC patients, but also the inflamed and non-inflamed colon of patients with active Crohn's disease (aCD) and the mucosa from patients with quiescent UC (qUC) or CD (qCD). c Mϕ subset composition, determined as in Fig. 1d, was also determined in the inflamed and non-inflamed mucosa from an aUC patient. Pooled data from several independent patients are shown in d. e HLA-DR levels, within each Mϕ subset, were determined in the inflamed and non-inflamed colonic mucosa from an aUC patient, the pooled data being from several independent experiments displayed in f. One-way ANOVA with Tukey correction was applied in b, while two-way ANOVA with Sidak correction was applied in d and f. Ad-hoc comparisons were performed, in all cases, compared with the healthy mucosa. p-Values < 0.05 were considered significant (*p < 0.05; **p < 0.01; ****p < 0.0001)
Given that it has been previously reported that HLA-DR expression is lower in intestinal Mϕ from inflamed IBD patients,22,23 we also studied whether this decreased expression was associated with any particular subset. Our results revealed that HLA-DR expression was decreased even further in CD11chigh mucosal monocyte-like cells (Fig. 5e), rendering, therefore, the inflamed mucosa from active IBD patients with lower HLA-DR expression on the CD11chigh (both in UC and CD) and the CD11cdim (just in UC) subsets with no differences in the HLA-DR expression on the CD11c− Mϕ-like subset (Fig. 5f).
CD11c− GI-Mϕ are derived from CD14+ monocytes
The phenotypical and functional characterization of GI-Mϕ subsets suggests that they may represent different maturation status of the same cell type. Hence, and similar to the murine Ly6C waterfall, monocytes may infiltrate the human mucosa as pro-inflammatory CD11chighCCR2+CX3CR1+ monocyte-like cells which, once in the mucosa, would be conditioned by the microenvironment to become transient CD11cdimCCR2lowCX3CR1low cells and finally tolerogenic CD11c−CCR2−CX3CR1− Mϕ. In order to assess this, we first determined whether Mϕ subsets changed their relative proportions following culture. Our results shown that the proportion of CD11chigh Mϕ was decreased just after 18 h (Fig. 6a). Moreover, if the culture was performed in the presence of the TLR4 ligand LPS, the swap in the Mϕ subset proportions was enhanced as it further decreased the proportion of the CD11chigh monocyte-like subset coupled with an expansion of the CD11c− Mϕ-like subset (Fig. 6b).
CD11c− macrophages are induced by the colonic mucosa. a Macrophage (Mϕ) colonic subset composition was determined as in Fig. 1d within fresh lamina propria mononuclear cells (LPMC), as well as after 18-h culture in complete medium (Basal). b Mϕ subset composition was also determined following 18-h culture of LPMC in the presence/absence of LPS. c GM-CSF-derived Mϕ were generated, following 7 days differentiation of CD14+ monocytes, in the presence/absence of cell-free colonic biopsy culture supernatant (Bx-SN). The proportion of CD11c− macrophages was determined at the end of the culture, as well as d the intracellular cytokine profile (IL-1β and IL-10) of both the CD11c+ and the CD11c− fractions. Two-way ANOVA repeated measures with Sidak correction was applied in a, b and d, while paired t-test was applied in c. p-Values <0.05 were considered significant (*p < 0.05; **p < 0.01; ***p < 0.001). Note that the 18-h plot in a and the 18-h basal culture plot in b are the same, as in a the plot is compared with their paired non-cultured cells, while in b the plot is compared with the paired 18-h culture in the presence of LPS
To further confirm that CD11c− Mϕ are derived from the mucosal infiltrating CD14+ monocytes, we next generated granulocyte-macrophage colony-stimulating factor (GM-CSF)-derived Mϕ in the presence of intestinal cell-free culture supernatants, or secretomes, which provide a source of intestinal microenvironments. While there was a residual proportion of naturally occurring CD11c− Mϕ following GM-CSF conditioning, their proportion was higher if the differentiation was performed in the presence of colonic secretomes from healthy controls (Fig. 6c). Moreover, these de novo generated CD11c− Mϕ displayed a tolerogenic IL-10-biased cytokine profile, as opposed to their classical IL-1β-producing CD11c+ counterparts (Fig. 6d). Together, our results suggest that CD11c− Mϕ are generated from CD14+ precursors following mucosal conditioning in the GI-tract.
Enhanced infiltration of circulating monocytes towards the IBD mucosa in a CCR2+-dependent manner
Given that CD11c− Mϕ are derived from circulating CD14+ monocytes following mucosal conditioning, we finally aimed to identify the mechanisms mediating monocyte migration towards the colon. To that end, we used transwell migration assays where we determined monocyte migration towards intestinal secretomes. Our results revealed that the colonic mucosa from healthy controls attracted circulating monocytes compared with the basal or spontaneous migration (data not shown). Indeed, such recruitment capacity was increased in the inflamed mucosa from IBD patients, either UC or CD, as compared with the healthy colonic mucosa (Fig. 7a). These results are consistent, therefore, with the higher numbers of pro-inflammatory CD11chigh monocyte-like cells found in the inflamed IBD mucosa (Fig. 5d). Moreover, monocyte recruitment was CCR2 dependent as migration was abrogated in all cases if CCR2 had been blocked on the monocytes prior to migration (Fig. 7b). Consequently, our results reveal that factors within the human colonic mucosa attract CD14+ monocytes via CCR2, and that this process is exacerbated in the inflamed mucosa in patients with active IBD. Thus, the increased infiltration of CD11chigh monocyte-like cells in the IBD mucosa is likely to be in response to inflammatory factors within the intestinal environment in IBD.
The inflamed colon from IBD patients displays an increased capacity to recruit circulating monocytes in a CCR2-dependent manner. a Colonic biopsy culture supernatants from healthy controls (HC), patients with active ulcerative colitis (aUC), quiescent ulcerative colitis (qUC), active Crohn's disease (aCD) or quiescent Crohn's disease (qCD) were further evaluated on transwell inserts for their capacity to recruit circulating CD14+ classical monocytes from healthy controls. b Prior to performing the migration, monocytes were pre-incubated with anti-CCR2 (or its respective isotype) to evaluate whether monocyte migration towards the colonic culture supernatants was CCR2 dependent. Dotted lines denote basal, or spontaneous migration, of monocytes towards the lower chamber with unconditioned medium. One-way ANOVA with Tukey correction was applied in a, while two-way ANOVA repeated measures with Sidak correction was applied in b. p-Values < 0.05 were considered significant (*p < 0.05; ***p < 0.001; ****p < 0.0001)
Discussion
Here we have described how human GI-Mϕ can be divided into subsets based on the expression levels of the CD11c integrin and the chemokine receptors CCR2 and CX3CR1. Hence, pro-inflammatory monocyte-like cells can be identified as CD11chighCCR2+CX3CR1+ cells, a phenotype also shared by circulating CD14+ monocytes. On the contrary, tissue-resident tolerogenic Mϕ can be identified as CD11c−CCR2−CX3CR1− cells. Moreover, a transition phenotype between the two can be also found on the basis of CD11c expression with such a subset displaying an intermediate phenotype and function between the others. Therefore, and although for pragmatic reasons here we have focused on the characterization of the cells based on the differences displayed by the CD11c integrin, similar results should have been obtained if Mϕ had been divided into subsets based on the expression of CCR2 or CX3CR1. Moreover, here we have also described how CD11c− IL-10-producing Mϕ are likely derived from circulating monocytes following mucosal conditioning. Finally, we have also reported how monocytes are recruited by the GI mucosa in a CCR2-dependent manner, such recruitment being increased in patients with active IBD where there is an accumulation of mucosal CD11chigh Mϕ.
The presence of CD11c− Mϕ9,10,23 has been previously reported in the human mucosa. Indeed, our results are in agreement with the manuscript recently published by Bujko et al.25, who described the presence of four human intestinal Mϕ subsets (two subsets of CD11c+ Mϕ and two subsets of CD11c− Mϕ). Hence, the CD11chigh, CD11cdim and CD11c− Mϕ subsets characterized in this manuscript share several characteristics (including phenotype and function) with the Mϕ1, Mϕ2 and Mϕ3 subsets, respectively, described by Bujko et al.25 Moreover, Bujko et al. have also proved how Mϕ1 (likely our CD11chigh population) and Mϕ2 (likely our CD11cdim population) subsets represent newly arrived monocytes which are immediately conditioned by the surrounding microenvironment and, subsequently, differentiate towards Mϕ3 in the mucosa (our CD11c− population) or Mϕ4 in the submucosa. In agreement with that, our results also provide evidence that CD14+ monocytes infiltrate the mucosa as pro-inflammatory CD11chigh where they are conditioned to become tolerogenic CD11c− Mϕ. Supporting our claims, we have shown that CD11c− IL-10-producing Mϕ are likely derived from CD14+ monocytes following mucosal conditioning. This differentiation seems to be indeed a spontaneous phenomenon in the mucosa as direct lamina propria mononuclear cells (LPMC) culture results in the conversion of CD11chigh monocyte-like cells into CD11c− Mϕ, such process being enhanced if the culture is performed in the presence of LPS. However, we cannot discard the possibility that the increased proportion of CD11c− Mϕ following culture may be due to a preferential cell death of the CD11chigh subset following culture. The nature, however, of the factors mediating such conversion is nevertheless currently unknown as the lamina propria is a non-sterile environment carrying not just several cell types (including non-immune cells) but also mucus components and microbial products. Hence, among the several mucosal factors which may play a role in such conditioning, we cannot discard the role of TLR-agonists (including LPS) or immunomodulatory cytokines like TGF-β.26,27,28 Nevertheless, given the complexity of all the lamina propria interactions taking place in the cultures, it is likely that rather than being induced by a single factor, Mϕ conversion is the consequence of several mucosal factors cooperating on a synergistic manner. Current work from our lab is aiming to show some light into these mechanisms aiming to identify the specific factors mediating CD14+ monocyte conversion into CD11c− cells and whether this process is altered in IBD.
Our results also provide an explanation to the previously described accumulation of CD14+ immature Mϕ-producing pro-inflammatory cytokines in the inflamed IBD mucosa.18,19,20,21,22,23 Those cells are a consequence of the increased CCR2-dependent monocyte-recruitment capacity elicited by the inflamed mucosa in IBD, hence contributing to the pool of intestinal pro-inflammatory CD11chigh monocyte-like cells. Nevertheless, we have not identified the chemokines mediating such a recruitment as the chemokine/receptor interaction takes place in a promiscuous manner.29 Besides, it is also currently unknown whether the increased capacity to recruit circulating monocytes elicited by the inflamed IBD mucosa is mediated by the same chemokines than in health (although at higher doses) or, on the contrary, it is mediated by different factors.
Murine Ly6Chigh monocytes infiltrate the mucosa as CCR2+CX3CR1−cells to subsequently differentiate towards tissue-resident CCR2−CX3CR1+ Mϕ.10,11 Nevertheless, the same does not seem to be true in the human context as both circulating CD14+ monocytes and tissue monocyte-like cells are CD11chighCCR2+CX3CR1+ cells as opposed to their Mϕ-like counterparts which are CD11c−CR2−CX3CR1−. Thus, and although murine mature CX3CR1+ Mϕ have been considered as regulatory and essential to expand intestinal Tregs and maintain GI homoeostasis,12,14,15,16,17 the same may not be applied in humans as newly arrived monocytes seem to decrease CX3CR1 expression during their tissue-induced differentiation process. These results may reflect differences in the species under study since, as opposed to the murine models where RALDH2 activity is restricted to CD103+ dendritic cells (DC), human GI-Mϕ mediate that activity too.18,20 Moreover, while the αVβ8 integrin (required to active latent TGF-β) in the murine GI tract is expressed by type 1 conventional DC,30 in humans it is, however, expressed by type 2 conventional DC.31 Together these observations confirm that despite their similarities, murine and human immune systems may display some important differences32,33 as we have addressed in this study.
In summary, in this study, we have described how human GI-Mϕ can be divided into pro-inflammatory monocyte-like CD11chighCCR2+CX3CR1+ cells and tissue-resident Mϕ-like CD11c−CR2−CX3CR1− cells, with CD11cdim cells displaying a transition phenotype between the two. Indeed, the inflamed mucosa from IBD patients carries higher numbers of CD11chigh monocyte-like cells, but not the others, and at the same time elicits an increased capacity to recruit circulating CD14+ monocytes in a CCR2-dependent manner. Unravelling the mechanisms mediating monocyte migration towards the inflamed mucosa as well as the identification of the mucosal factors mediating monocyte differentiation towards tolerogenic Mϕ may identify novel targets to perform tissue-specific immunomodulation in IBD, aiming to restore the altered immune response.
Methods
Patients and biological samples
Intestinal biopsies from healthy controls were obtained during colonoscopy or endoscopy from a total of 52 healthy controls (37.1% males; 52.4 ± 12.4 years (mean ± standard deviation); age interval 25–80). Patients had been referred due to rectal bleeding, dyspepsia or colorectal cancer screening. In all cases, they had macroscopically and histologically normal mucosa. In the case of a colonoscopy, paired samples were obtained from the distal colon, proximal colon and the terminal ileum from the same patients. Duodenal samples were obtained in the context of an endoscopy. A maximum of eight biopsies were obtained per tissue/patient. Samples were immediately preserved in ice-chilled complete medium (Dutch modified RPMI 1640 (Sigma-Aldrich, Dorset, UK) containing 100 µg/mL penicillin/streptomycin, 2 mM L-glutamine, 50 µg/mL gentamicine (Sigma-Aldrich) and 10% foetal calf serum (TCS cellworks, Buckingham, UK)) and processed within 30 min. Colonic biopsies from IBD patients, including patients with active UC (defined by a Mayo endoscopic score >1; Supplementary Table 1), quiescent UC (defined by a Mayo endoscopic score ≤1; Supplementary Table 2), active CD (defined by a simplified endoscopic activity score for CD (SES-CD) score >3; Supplementary Table 3) or quiescent CD (defined by a SES-CD score ≤3; Supplementary Table 4), were sampled and processed in the same manner as those from the healthy controls. In the case of patients with active disease (either UC or CD), both the inflamed and the non-inflamed colonic mucosa were sampled. GI-Mϕ characterization was exclusively performed using samples obtained at La Princesa Hospital, while biopsy cultures to obtain intestinal secretomes were performed both at La Princesa and at the University Mútua Terrassa Hospitals. Blood samples were also obtained from the same patients subjected to colonoscopy as well as from healthy volunteers with no known autoimmune disease or malignancy. In all cases, samples were obtained following informed consent after ethical approval from the Ethics Committee at La Princesa Hospital or the University Mútua Terrassa Hospital.
Blood processing
Peripheral blood mononuclear cells (PBMC) were obtained by centrifugation over Ficoll-Paque PLUS (Amersham Biosciences, Chalfont St. Giles, UK). PBMC were washed twice in PBS containing 1 mM EDTA and 0.02% sodium azide (FACS buffer) and stained with fluorochrome-conjugated antibodies as explained below. In other cases, total PBMC were used to enrich CD14+ monocytes (purities over 95% in all the cases) using magnetically labelled microbeads (Miltenyi Biotec) following manufacturer's instructions.
Biopsy processing
Intestinal biopsies were processed as previously described.34 In some cases, freshly obtained intestinal biopsies were immediately cultured in the complete medium (1 biopsy per 0.5 mL of complete medium per well in 24-well culture plates) for 18 h after which the complete medium was harvested, centrifuged and the cell-free culture supernatants cryopreserved (−80 °C) until used. In other cases, intestinal biopsies were processed to obtain LPMC following two incubations (30 min each) with Hanks balanced salt solution (HBSS) (Gibco BRL, Paisley, Scotland, UK) containing 1 mM DTT and 1 mM EDTA solutions to remove the associated mucus/bacteria and epithelial layer, respectively, and further digested in the presence of 1 mg/mL of collagenase D and 20 μg/mL of liberase (Roche Diagnostics Ltd., Lewes, UK). LPMC were subsequently passed through a 100-μm cell strainer and collected by centrifugation before they were further used for flow cytometry staining or culture (5 million LPMC in 2.5 mL of complete medium) in the presence/absence of LPS (100 ng/mL, Sigma-Aldrich).
Mϕ differentiation
Classical blood-derived Mϕ were obtained following differentiation of CD14+ enriched monocytes. Briefly, purified monocytes were cultured for 7 days (100,000 cells in 100 μL in 96 flat-bottom culture plates) in ImmunoCultTM-SF Macrophage medium (StemCell Technologies) supplemented with 100 ng/mL of GM-CSF (Miltenyi), which was freshly replenished on day 3. Cell-free colonic culture supernatants were defrosted and further centrifuged to remove any debris. These culture supernatants, which provide a source of intestinal microenvironments, were further added to the macrophage differentiation medium during the whole differentiation process (1:1 ratio) to assess their capacity to induce of CD11c− Mϕ.
Transwell migration experiments
Cell-free colonic culture supernatants, processed as previously described, were placed in the lower chamber of transwell inserts to determine the mucosal capacity to recruit circulating monocytes. To that end, 200,000 CD14+ monocytes were seeded in the upper insert of transwell culture plates (Corning) and their capacity to migrate through 3 μm pores for a period of 4 h was assessed by flow cytometry following the collection of the cells in the lower chamber. In some experiments, monocytes were incubated with anti-CCR2 (R&D Systems), or its respective isotype, prior to performing migration experiments. In all cases, results were relativized with the spontaneous migration of the cells determined as the total numbers of migrated monocytes towards not-supplemented unconditioned complete medium.
Antibody labelling
PBMC, LPMC, monocytes or Mϕ were stained with monoclonal antibodies and characterized by flow cytometry. In all cases, a Live/Dead fixable near-IR dead cell stain kit (Molecular Probes) was added to the cells prior to performing antibody staining, hence allowing the exclusion of dead cells from the analysis. Supplementary Table 5 shows the specificity, clone, fluorochrome and sources of the antibodies used. Cells were labelled in FACS buffer on ice and in the dark for 20 min following Fc block incubation (Becton Dickinson). For the assessment of intracellular cytokines, the cells were permeabilized (Leucoperm, Abd Secrotec) following surface staining and stained with intracellular antibodies. In all cases, cells were further washed in FACS buffer, fixed with 2% paraformaldehyde in FACS buffer for 10 min on ice, and washed again in FACS buffer before they were stored at 4 °C prior to acquisition on the flow cytometer.
Flow cytometry and data analysis
Cells were acquired either on a LSR-Fortessa (BD Biosciences) for monocoyte/Mϕ characterization or on a BD Canto II flow cytometer (BD Biosciences) for monocyte migration assays. In all cases, the results were analyzed using FlowJow (version 10.1). All cells were analyzed within the singlet viable fraction. Positive and negative gatings were set by the FMO method.
Statistical analysis
A t-test or one/two-way ANOVA (with our without repeated measures) and subsequent Tukey or Sidak ad-hoc correction were applied as detailed in each figure legend. The level of significance was fixed at p < 0.05 in all cases.
References
Gomollón, F. et al. 3rd European evidence-based consensus on the diagnosis and management of Crohn's disease 2016: Part 1: diagnosis and medical management. J. Crohns Colitis 11, 3–25 (2017).
Magro, F. et al. Third european evidence-based consensus on diagnosis and management of ulcerative colitis. Part 1: definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery, and ileo-anal pouch disorders. J. Crohns Colitis 11, 649–670 (2017).
Rogler, G. Resolution of inflammation in inflammatory bowel disease. Lancet Gastroenterol. Hepatol. 2, 521–530 (2017).
Molodecky, N. A. et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 142, 46–54.e42 (2012).
Gisbert, J. P., Marín, A. C., McNicholl, A. G. & Chaparro, M. Systematic review with meta-analysis: the efficacy of a second anti-TNF in patients with inflammatory bowel disease whose previous anti-TNF treatment has failed. Aliment. Pharmacol. Ther. 41, 613–623 (2015).
Lavin, Y. et al. Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell 159, 1312–1326 (2014).
Persson, E. K., Scott, C. L., Mowat, A. M. & Agace, W. W. Dendritic cell subsets in the intestinal lamina propria: ontogeny and function. Eur. J. Immunol. 43, 3098–3107 (2013).
Cerovic, V., Bain, C. C., Mowat, A. M. & Milling, S. W. F. Intestinal macrophages and dendritic cells: what's the difference? Trends Immunol. 35, 270–277 (2014).
Bain, C. C. & Mowat, A. M. Macrophages in intestinal homeostasis and inflammation. Immunol. Rev. 260, 102–117 (2014).
Bain, C. C. et al. Resident and pro-inflammatory macrophages in the colon represent alternative context-dependent fates of the same Ly6Chi monocyte precursors. Mucosal Immunol. 6, 498–510 (2013).
Bain, C. C. et al. Constant replenishment from circulating monocytes maintains the macrophage pool in the intestine of adult mice. Nat. Immunol. 15, 929–937 (2014).
Joeris, T., Müller-Luda, K., Agace, W. W. & Mowat, A. M. Diversity and functions of intestinal mononuclear phagocytes. Mucosal Immunol. 10, 845–864 (2017).
Zigmond, E. & Jung, S. Intestinal macrophages: well educated exceptions from the rule. Trends Immunol. 34, 162–168 (2013).
Mowat, A. M. & Bain, C. C. Mucosal macrophages in intestinal homeostasis and inflammation. J. Innate Immun. 3, 550–564 (2011).
Tamoutounour, S. et al. CD64 distinguishes macrophages from dendritic cells in the gut and reveals the Th1-inducing role of mesenteric lymph node macrophages during colitis. Eur. J. Immunol. 42, 3150–3166 (2012).
Zigmond, E. et al. Ly6C hi monocytes in the inflamed colon give rise to proinflammatory effector cells and migratory antigen-presenting cells. Immunity 37, 1076–1090 (2012).
Rivollier, A., He, J., Kole, A., Valatas, V. & Kelsall, B. L. Inflammation switches the differentiation program of Ly6Chi monocytes from antiinflammatory macrophages to inflammatory dendritic cells in the colon. J. Exp. Med. 209, 139–155 (2012).
Sanders, T. J. et al. Increased production of retinoic acid by intestinal macrophages contributes to their inflammatory phenotype in patients with Crohn's disease. Gastroenterology 146, 1278–1288 (2014).
Thiesen, S. et al. CD14(hi)HLA-DR(dim) macrophages, with a resemblance to classical blood monocytes, dominate inflamed mucosa in Crohn's disease. J. Leukoc. Biol. 95, 531–541 (2014).
Magnusson, M. K. et al. Macrophage and dendritic cell subsets in IBD: ALDH+cells are reduced in colon tissue of patients with ulcerative colitis regardless of inflammation. Mucosal Immunol. 9, 171–182 (2016).
Kühl, A. A., Erben, U., Kredel, L. I. & Siegmund, B. Diversity of Intestinal macrophages in inflammatory bowel diseases. Front. Immunol. 6, 613 (2015).
Dige, A. et al. Reduced numbers of mucosal DR(int) macrophages and increased numbers of CD103(+) dendritic cells during anti-TNF-α treatment in patients with Crohn's disease. Scand. J. Gastroenterol. 51, 692–699 (2016).
Matsuno, H. et al. CD103+ dendritic cell function is altered in the colons of patients with ulcerative colitis. Inflamm. Bowel Dis. 23, 1524–1534 (2017).
Mowat, A. M. & Agace, W. W. Regional specialization within the intestinal immune system. Nat. Rev. Immunol. 14, 667–685 (2014).
Bujko, A. et al. Transcriptional and functional profiling defines human small intestinal macrophage subsets. J. Exp. Med. 215, 441–458 (2018).
Smythies, L. E. et al. Inflammation anergy in human intestinal macrophages is due to Smad-induced IkappaBalpha expression and NF-kappaB inactivation. J. Biol. Chem. 285, 19593–19604 (2010).
Maheshwari, A. et al. TGF-β2 suppresses macrophage cytokine production and mucosal inflammatory responses in the developing intestine. Gastroenterology 140, 242–253 (2011).
Schridde, A. et al. Tissue-specific differentiation of colonic macrophages requires TGFβ receptor-mediated signaling. Mucosal Immunol. 10, 1387–1399 (2017).
Blanchet, X., Langer, M., Weber, C., Koenen, R. R. & von Hundelshausen, P. Touch of chemokines. Front. Immunol. 3, 175 (2012).
Luda, K. M. et al. IRF8 transcription-factor-dependent classical dendritic cells are essential for intestinal T cell homeostasis. Immunity 44, 860–874 (2016).
Fenton, T. M. et al. Inflammatory cues enhance TGFβ activation by distinct subsets of human intestinal dendritic cells via integrin αvβ8. Mucosal Immunol. 10, 624–634 (2017).
Gibbons, D. L. & Spencer, J. Mouse and human intestinal immunity: same ballpark, different players; different rules, same score. Mucosal Immunol. 4, 148–157 (2011).
Mann, E. R. et al. Intestinal dendritic cells: their role in intestinal inflammation, manipulation by the gut microbiota and differences between mice and men. Immunol. Lett. 150, 30–40 (2013).
Bernardo, D. et al. Chemokine (C-C motif) receptor 2 mediates dendritic cell recruitment to the human colon but is not responsible for differences observed in dendritic cell subsets, phenotype, and function between the proximal and distal colon. Cell. Mol. Gastroenterol. Hepatol. 2, 22–39.e25 (2016).
Acknowledgements
The authors kindly thank the critical input and suggestions by Dr. Elizabeth R Mann and Dr. William W Agace on this study. This work was supported by the Spanish Ministry of Economy (SAF2014−56642-JIN); the Instituto de Salud Carlos III (PIE13/00041, EHD16PI02); the “Asociación Española de Gastroenterología” (Becas Nuevos Investigadores 2016 and 2017) and the Community of Madrid (Consejería de Educación, Juventud y Deporte, Programa de Garantía Juvenil 2015 and 2016).
Author information
Authors and Affiliations
Contributions
D.B. was involved with study concept and design, experimental procedures, analysis and interpretation of the data and statistical analysis. I.M.G., A.C.M., A.M.A., S.F.T., A.D.G., A.C., E.T. and L.O.M. were involved with experimental procedures together with data analysis and interpretation. R.C.F., P.M., F.C., M.C., M.J., F.D.l.M., C.S., M.E., M.C. and J.P.G. performed patients identification and recruitment as well as obtention of all biological samples. D.B. and J.P.G. obtained the funds to perform this work. The manuscript was drafted by D.B. and edited by D.B., A.C.M., A.M.A., S.F.T., M.E., M.C. and J.P.G. All authors reviewed and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Bernardo, D., Marin, A.C., Fernández-Tomé, S. et al. Human intestinal pro-inflammatory CD11chighCCR2+CX3CR1+ macrophages, but not their tolerogenic CD11c−CCR2−CX3CR1− counterparts, are expanded in inflammatory bowel disease. Mucosal Immunol 11, 1114–1126 (2018). https://doi.org/10.1038/s41385-018-0030-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41385-018-0030-7
This article is cited by
-
Macrophage polarization toward M1 phenotype in T cell transfer colitis model
BMC Gastroenterology (2023)
-
Diesel Exhaust Particle (DEP)-induced glucose intolerance is driven by an intestinal innate immune response and NLRP3 activation in mice
Particle and Fibre Toxicology (2023)
-
Integration of taxa abundance and occurrence frequency to identify key gut bacteria correlated to clinics in Crohn’s disease
BMC Microbiology (2023)
-
Macrophages in intestinal homeostasis and inflammatory bowel disease
Nature Reviews Gastroenterology & Hepatology (2023)
-
New insights into muscularis macrophages in the gut: from their origin to therapeutic targeting
Immunologic Research (2023)