Abstract
Asthmatics sensitized to fungi are reported to have more severe asthma, yet the immunopathogenic pathways contributing to this severity have not been identified. In a pilot assessment of human asthmatics, those subjects sensitized to fungi demonstrated elevated levels of the common γ-chain cytokine IL-7 in lung lavage fluid, which negatively correlated with the lung function measurement PC20. Subsequently, we show that IL-7 administration during experimental fungal asthma worsened lung function and increased the levels of type 2 cytokines (IL-4, IL-5, IL-13), proallergic chemokines (CCL17, CCL22) and proinflammatory cytokines (IL-1α, IL-1β). Intriguingly, IL-7 administration also increased IL-22, which we have previously reported to drive immunopathogenic responses in experimental fungal asthma. Employing IL22CreR26ReYFP reporter mice, we identified γδ T cells, iNKT cells, CD4 T cells and ILC3s as sources of IL-22 during fungal asthma; however, only iNKT cells were significantly increased after IL-7 administration. IL-7-induced immunopathogenesis required both type 2 and IL-22 responses. Blockade of IL-7Rα in vivo resulted in attenuated IL-22 production, lower CCL22 levels, decreased iNKT cell, CD4 T-cell and eosinophil recruitment, yet paradoxically increased dynamic lung resistance. Collectively, these results suggest a complex role for IL-7 signaling in allergic fungal asthma.
Similar content being viewed by others
Introduction
Among severe asthmatics, sensitivity to fungi range from 25% to over 70% (reviewed in ref. 1). Among these organisms, the most common species include Alternaria, Cladosporium, Penicillium and Aspergillus.1 Indeed, a recent review of longitudinal studies assessing increased exposure to indoor fungi before the development of asthma symptoms suggests that species of Penicillium, Aspergillus and Cladosporium pose a respiratory health risk in susceptible populations.2 Moreover, increased exacerbation of current asthma symptoms in children and adults were associated with increased levels of Penicillium, Aspergillus, Cladosporium and Alternaria species.2 Asthma in many of these individuals is now recognized as severe asthma with fungal sensitization, which is considered a specific asthma phenotype.3,4 Several studies have shown that sensitization to one or more fungal organisms correlate with hospital/intensive care unit admissions of severe asthmatics compared to asthmatics not requiring hospitalization.5,6 Other studies have reported that Aspergillus-sensitized asthmatics with specific IgE had lower lung function, more bronchiectasis and higher sputum neutrophil numbers,7 whereas an additional study has shown that these asthmatics have higher steroid usage compared to asthmatics who are not sensitized.8 Finally, simple sensitization to Aspergillus fumigatus may have dramatic effects on lung function in individuals with cystic fibrosis9,10 and chronic obstructive pulmonary disease,11 thus extending the importance of A. fumigatus beyond invasive infection and asthma.
We have previously reported that beta-glucan recognition via the beta-glucan receptor Dectin-1 drove immunopathogenic responses during experimental fungal asthma.12 Specifically, mice deficient in Dectin-1 had significantly better lung function, significantly lower Muc5ac and Clca3 (Gob5) lung messenger RNA expression, significantly lower proallergic CCL17/TARC, CCL22/MDC and IL-33 lung levels and significantly lower proinflammatory interleukin (IL)-1β and CXCL1/KC levels as well as neutrophil levels.12 Intriguingly, the entire Dectin-1-deficient phenotype was recapitulated by deficiency in a single cytokine, the IL-10 family cytokine IL-22. Moreover, neutralization of IL-22 during experimental fungal asthma improved lung function.12 To date, many cell types, including CD4+ T cells, CD8+ T cells, γδ T cells, natural killer (NK) cells, invariant NKT (iNKT) cells, LTi cells and type three innate lymphoid cells (ILC3s) are known sources of IL-22 (reviewed in ref. 13). In our previous work, CD4+ T cells from Dectin-1-deficient mice demonstrated significantly lower production of T helper type 1, 2 and 17 (Th1, Th2 and Th17) cytokines, but not IL-22.12 In fact, IL-22 was primarily produced by unfractionated lung digest cells in a Dectin-1-dependent manner, suggesting a non-CD4+ T-cell source of IL-22. In the current body of work, we found that the common γ-chain cytokine IL-7 was elevated in human asthmatics who were sensitized to fungi compared to subjects who were atopic but not fungal sensitive, with the levels of IL-7 negatively correlating to lung function. As IL-7 supports the development of many of the cell types that may produce IL-22, we sought to further define a role for IL-7 in immunopathogenesis during fungal asthma.
Results
EGF, IL-1RA and IL-7 levels are significantly elevated in BALF from human asthmatics who are sensitized to fungi and negatively correlate with lung function
In an effort to better understand fungal asthma severity in adult asthmatics, we analyzed bronchoalveolar lavage fluid (BALF) from adult asthmatics who were atopic and sensitized to fungi vs. asthmatics who were atopic but not sensitized to fungi via a Luminex®-based multiplex protein suspension array. Fungal (+) asthmatics were 83% positive for Alternaria, 41% positive for Aspergillus and 21% positive for Cladosporium. Cladosporium sensitivity always combined with either Alternaria or Aspergillus sensitivity (i.e., not observed as a lone positive), 7% of subjects were positive for both Alternaria and Aspergillus and 17% of subjects were positive for all three fungi. Demographics and clinical characteristics for these subjects are presented in Table 1. Fungal (+) asthmatics demonstrated lower FEV1 compared to fungal (−) (74.9 vs. 83.2%; P = 0.021) and lower Forced expiratory volume in one second (FEV1)/forced vital capacity (FVC) ratio (0.696 vs. 0.76; P = 0.002) indicative of a moderate persistent phenotype (Table 1). Moreover, a greater maximal FEV1 reversal was observed after albuterol treatment in fungal (+) asthmatics compared to fungal (−) (25.2 vs. 8.06%; P < 0.0001), whereas a significantly lower logPC20 (PC20 = concentration of methacholine producing 20% drop in FEV1) was observed (−0.49 vs. 0.055; P = 0.032; Table 1). Fungal (+) asthmatics also demonstrated higher IgE, blood eosinophil and exhaled nitric oxide levels (P = 0.0043, 0.005 and 0.049, respectively; Table 1). There were no differences in age, asthma duration and body mass index between fungal (+) and fungal (−) asthmatics (Table 1), nor were there differences in sex, race or ethnicity (data not shown). There were also no differences in short acting β-agonist usage (89.7 vs. 96.7%, fungal (+) vs. fungal (−), P = 0.353), long acting β-agonist usage (65.5 vs. 53.3%, P = 0.492), leukotriene receptor antagonist usage (20.7 vs. 16.7%, P = 0.95) and inhaled corticosteroid usage (79.3 vs. 53.3%, P = 0.067). There was no difference in sensitivity to cockroach or house dust mite between fungal (+) and fungal (−) asthmatics, but fungal (+) asthmatics were more sensitive to cats, ragweed and trees (data not shown). From the Luminex® analysis, we observed 12 mediators than were significantly higher in fungal (+) asthmatics compared to fungal (−) asthmatics: epidermal growth factor (EGF), transforming growth factor-α (TGF-α), CCL11, CCL15, CCL17, CX3CL1, granulocyte-macrophage colony-stimulating factor (GM-CSF), TRAIL (tumor necrosis factor (TNF)-related apoptosis-inducing ligand), IL-1β, IL-1RA, IL-5 and IL-7 (Table 2). PC20, also known as the methacholine challenge test, is an essential measurement of airway hyperreactivity.14 Table 1 demonstrated that fungal (+) asthmatics required a much lower dose of methacholine to negatively affect FEV1 (PC20), which supports the contention than fungal (+) asthmatic have more severe asthma. Therefore, we performed regression analysis to determine whether a significant association existed between the 12 mediators that were elevated in fungal (+) asthmatics and PC20 and then subsequently performed correlation analysis to determine whether the correlation was positive or negative. We found that higher levels of EGF (Fig. 1a), IL-1RA (Fig. 1b) and IL-7 (Fig. 1c) in fungal (+) asthmatics had a significant negative association with bronchial hyperresponsiveness to methacholine (Fig. 1d–f). EGF is relatively well studied in asthma pathogenesis, including fungal asthma, where experimental models of Alternaria15 and Aspergillus16 have documented upregulation of the EGF receptor and its various ligands. The observation of high IL-1RA levels negatively correlating with PC20 is intriguing and one we are currently pursuing (Godwin and Steele, manuscript in preparation). IL-7 however was an unexpected finding based on the acknowledged challenges associated with determining IL-7 bioavailability.17 Overall, these data reveal that elevated IL-7 levels in human asthmatics sensitized to fungi are associated with increased airway hyperesponsiveness.
EGF, IL-1RA and IL-7 levels are significantly elevated in in bronchoalveolar lavage fluid from human asthmatics that are sensitized to fungi and negatively correlate with lung function. Bronchoalveolar lavage fluid was collected from subjects with atopic asthma who were or were not sensitive to fungi. a EGF (n = 30 and 28 for fungal (−) vs. fungal (+)), b IL-1RA (n = 29 and 29 for fungal (−) vs. fungal (+)) and c IL-7 (n = 30 and 29 for fungal (−) vs. fungal (+)) levels were quantified in clarified, concentrated supernatants by Bio-Plex. Data were normalized to the total protein concentration of each sample. Data are expressed as pg/mg protein (each symbol represents a single subject). Pearson's coefficient for (d) EGF and PC20, e IL-1RA and PC20 and f IL-7 and PC20 in fungal (+) subjects (n = 26 for each cytokine)
IL-7 administration promotes worse lung function in the presence of increased type 2, proinflammatory and IL-22 responses during experimental fungal asthma
As IL-7 levels were elevated in human asthmatics sensitized to fungi and correlated with worse lung function, we sought to determine how IL-7 contributes to asthma severity. Mice deficient in IL-7 do not develop fungal asthma as a result of significant developmental defects in many lymphocyte populations associated with asthma immunopathogenesis (data not shown). Therefore, we administered IL-7 over the course of an experimental model of fungal asthma12 (Fig. 2a) and assessed lung function. IL-7 administration during fungal asthma resulted in a profound increase in dynamic lung resistance compared to mice that received vehicle (phosphate-buffered saline (PBS)) over the course of experimental fungal asthma (Fig. 2b). IL-7 administration alone in the absence of chronic A. fumigatus exposure resulted in lung resistance similar to that we have previously reported for naive mice12 (Supplemental Fig. 1). As we have previously reported that experimental fungal asthma is a mix of type 2 and proinflammatory responses,12 we determined whether these responses were affected by IL-7 administration. Indeed, we observed significant elevations in the type 2 cytokines IL-5 and IL-13 (Fig. 2c) (IL-4 was also significantly higher, data not shown) coupled with increases in the proallergic chemokines CCL17 and CCL22 (Fig. 2d). Mice administered IL-7 also had significant increases in IL-1α, IL-1β and TNF-α (Fig. 2e). In conjunction with increases in type 2 and proinflammatory cytokines, we further demonstrate that IL-7 treatment substantially increased the absolute number of CD4+ T cells and eosinophils, but not neutrophils (Fig. 2f). As we have previously reported that IL-22 contributes to fungal asthma,12 we questioned whether the administration of IL-7 affected the induction of IL-22. Results in Fig. 2g show that lung cells from mice administered IL-7 produced fourfold higher levels of IL-22 than those from mice receiving vehicle. IL-7 administration alone in the absence of chronic A. fumigatus exposure did not affect IL-22 induction (258 ± 121, 655 ± 28 and 1147 ± 148 pg/ml of IL-22 for IL-7 alone, chronic A. fumigatus and chronic A. fumigatus+IL-7, respectively). Thus, IL-7 drives immunopathogenic responses during fungal asthma, putatively via the induction of type 2 cytokines and IL-22.
IL-7 administration promotes worse lung function in the presence of increased type 2, proinflammatory and IL-22 responses during experimental fungal asthma. a C57BL/6 mice were subjected to experimental fungal asthma, and on days 7, 9, 11 and 14 intratracheally administered recombinant mouse IL-7 or vehicle. b At 24 h after the last organism challenge, dynamic lung resistance was analyzed via mechanical ventilation using the flexiVent system. The Figure illustrates cumulative data from two independent studies. Data expressed as mean ± SEM. c, d, e At 24 h after the last organism challenge, the right lungs were collected, enzymatically digested and unfractionated lung cells cultured for 24 h in the presence of A. fumigatus conidia or the right lungs were collected, homogenized and clarified by centrifugation. c IL-5 and IL-13 levels in lung digest cell culture supernatants, d CCL17 and CCL22 levels in lung homogenates and (e) IL-1α, IL-1β and TNF-α levels in lung digest cell culture supernatants were quantified by ELISA or Bio-Plex. The Figures illustrates cumulative data from two to three independent studies. f At 24 h after the last organism challenge, lung cells were isolated by bronchoalveolar lavage, enumerated, Fc-blocked and stained with a live/dead staining kit followed by staining with fluorochrome-conjugated antibodies corresponding to the various cell populations. The Figure illustrates cumulative data from two to three independent studies. g At 24 h after the last organism challenge, the right lungs were collected, enzymatically digested and unfractionated lung cells cultured for 24 h in the presence of A. fumigatus conidia. IL-22 levels were quantified in clarified co-culture supernatants by ELISA. The Figure illustrates cumulative data from two to three independent studies. For all graphs, *, ** and ***, represent P values of <0.05, <0.01 and < 0.001, respectively; n = 4–6 mice/group for each study; each data point/dot represents a single mouse and the line in each group corresponds to the mean (b–f)
IL-22 cell sources during experimental fungal asthma
As mice treated with IL-7 had a dramatic increase in IL-22 production, and the receptor for IL-7 (CD127) is expressed on many of the cell types that produce IL-22,68 we sought to determine the lung cell sources of IL-22. IL-22CreR26ReYFP mice were generated and subjected to experimental fungal asthma alongside control (PBS) treated animals (Fig. 3a) and analyzed for enhanced yellow fluorescent protein (eYFP) expression. The results in Fig. 3a show lung digest cells from experimental fungal asthma mice have significantly more eYFP+ (IL-22+) cells compared to control mice (4932 ± 403 in control vs. 12,460 ± 897 in fungal asthma). After extensive analysis, we were able to exclude CD11b+, CD11c+, NK1.1+, and CD8+ cells as significant sources of IL-22 in fungal asthma (Supplemental Fig. 2a). However, we identified the following cell types as IL-22 producers: γδ T cells (represented as γδ TCR+), CD4 T cells (represented as CD4+), iNKT cells (represented as PBS57-loaded CD1d tetramer+), and a fourth population that was negative for all markers shown (Fig. 3a). Upon further analyses, we determined that this fourth eYFP+/IL-22+ population is ILC3s (Supplemental Fig. 2B). This population was found to be Lin-CD45+Thy1+eYFP+ (lineage gate includes: CD11b, CD11c, CD3, CD4, CD8a, CD19, Gr1, FcεR1, KLRG1, γδ TCR and NKp46). While we observed all of these populations (γδ T cells, iNKT cells, CD4 T cells and ILC3s) expressing eYFP (IL-22) in both control (Fig. 3a, top panels) and fungal asthma (Fig. 3a, bottom panels), it is interesting to note the shift in cell population distribution upon exposure to fungal asthma. After fungal exposure, there was significant expansion of IL-22-producing CD4 T cells (440 ± 63 control vs. 1440 ± 275 fungal asthma, P = 0.0239) and iNKTs (96 ± 22 control vs. 3027 ± 750 FA, P = 0.0175) (Fig. 3a). However, no expansion of γδ T cells (2879 ± 663 control vs. 3981 ± 1229 FA, P = 0.2068) or ILC3s (1516 ± 762 control vs. 3013 ± 576 FA, P = 0.1920) was observed (Fig. 3a). Intriguingly, we observed that IL-7 administration over the development of fungal asthma in normal BL/6 mice led to significant increases in the total iNKT cell population (12,662 ± 2974 vehicle vs. 29,542 ± 6665 + rIL-7, P = 0.0360); however, again no changes were noted in the total γδ T-cell population (59,104 ± 13,622 vehicle vs. 44,911 ± 11,028 + rIL-7, P = 0.4235) (Fig. 3c). These data indicate that CD4 T cells and iNKTs may be the major cell source of IL-22 effected by a lung environment high in IL-7. We confirmed this by administering IL-7 to the IL-22CreR26ReYFP reporter mice (Fig. 3b). In mice treated with recombinant IL-7, eYFP+/IL-22+ iNKTs were increased approximately threefold (5878 ± 1719 vehicle vs. 18,983 ± 3932 + rIL-7, P = 0.0432). However, rIL-7-treated mice did not show a significant increases in eYFP+/IL-2+ CD4 T cells (203 ± 17 vehicle vs. 2128 ± 711 + rIL-7, P = 0.0709), γδ T cells (8318 ± 2355 vehicle vs. 9103 ± 1242 + rIL-7, P = 0.7611) or ILC3s (6205 ± 2630 vehicle vs. 9271 ± 3828 + rIL-7, P = 0.6343) (Fig. 3b). Thus, while multiple cell sources of IL-22 exist in the lung, exposure to A. fumigatus reshapes these populations and specifically expands the pool of IL-22-producing CD4 T cells and iNKTs. Furthermore, a lung environment high in IL-7 leads to an even greater expansion of IL-22-producing CD4 T cells and iNKTs, but not γδ T cells or ILC3s during fungal asthma.
IL-22 cell sources during experimental fungal asthma. a IL-22CreR26ReYFP reporter mice were subjected to experimental fungal asthma or a control exposure (PBS). At 24 h after the last organism challenge, the right lungs were collected, enzymatically digested and unfractionated lung cells cultured for 4 h in the presence of IL-1β. Lung cells were Fc-blocked, stained with a live/dead staining kit followed by staining with fluorochrome-conjugated antibodies corresponding to γδ T cells, iNKT cells, CD4 T cells and ILC3s. The Figure illustrates concatenated flow plots from a representative experiment. Results show the absolute cell numbers of eYFP+/IL-22+γδ T cells, iNKT cells, CD4 T cells and ILC3s. b IL-22CreR26ReYFP reporter mice were subjected to experimental fungal asthma and administered IL-7 or vehicle. At 24 h after the last organism challenge, the right lungs were collected, enzymatically digested and unfractionated lung cells cultured for 4 h in the presence IL-1β. Lung cells were Fc-blocked, stained with a live/dead staining kit followed by staining with fluorochrome-conjugated antibodies corresponding to γδ T cells, iNKT cells, CD4 T cells and ILC3s. Results show the absolute numbers of eYFP+/IL-22+γδ T cells, iNKT cells, CD4 T cells and ILC3s. The Figure illustrates concatenated flow plots from a representative experimental. c C57BL/6 mice were subjected to experimental fungal asthma and administered IL-7 as in (b). At 24 h after the last organism challenge, the right lungs were collected, enzymatically digested and unfractionated lung cells enumerated, Fc-blocked, stained with a live/dead staining kit and stained with fluorochrome-conjugated antibodies corresponding antibodies corresponding to iNKT cells and γδ T cells. The Figure illustrates cumulative data from two to three independent studies. Data are expressed as total number of iNKT cells and γδ T cells. *P value of <0.05; n = 4–6 mice/group for each study; each data point/dot represents a single mouse and the line in each group corresponds to the mean
The immunopathogenic effects of IL-7 during experimental fungal asthma require both type 2 and IL-22 responses
We have reported that the immune response generated during experimental fungal asthma does not favor a single T helper or innate response but rather results in broad induction of type 1/Th1, type 2/Th2, Th17, IL-22 and proinflammatory responses.12 As some of these responses were augmented by IL-7 treatment, we questioned whether IL-7-driven immunopathogenesis was primarily dependent on one response vs. another (i.e., type 2 vs. IL-22). Studies have previously shown that blocking ST2, the receptor for the pro-type 2 cytokine IL-33, ameliorated many pathological features of fungal asthma.18 To this end, we treated Il1rl1-/- mice (IL-33R/ST2, lack type 2 responses) or Il22-/- mice (lack IL-22 responses) with IL-7 as above and determined whether asthma severity, as measured by proallergic CCL17 and CCL22 levels, was modulated. CCL17 and CCL22 are considered diagnostic and potentially prognostic markers during allergic bronchopulmonary aspergillosis.19,20 Moreover, a previous report has shown that mice deficient in CCR4, the receptor for CCL17 and CCL22, had reduced airway hyperresponsiveness during fungal asthma,21 whereas we have previously reported that CCL17 and CCL22 levels served as markers of fungal asthma severity.12 Results show that compared to wild-type (WT) mice, CCL17 (Fig. 4a) and CCL22 (Fig. 4b) were both significantly reduced in Il1rl1-/- mice and Il22-/- mice as expected.12,18 Surprisingly however, IL-7 administration to fungus-challenged Il1rl1-/- mice did not result in attenuated production of either CCL17 (Fig. 4a) or CCL22 (Fig. 4b) when compared to fungus-challenged Il1rl1-/- mice that were administered vehicle, suggesting that IL-7-mediated immunopathogenesis was intact in the absence of type 2 responses. Likewise, IL-7-associated increases in CCL17 (Fig. 4a) and CCL22 (Fig. 4b) were not affected by the absence of IL-22. As type 2 responses or IL-22 responses alone are sufficient to drive immunopathogenesis in fungal asthma, these results suggest that IL-7-mediated immunopathogenesis requires simultaneously induction of both type 2/Th2 and IL-22 responses.
The immunopathogenic effects of IL-7 during experimental fungal asthma require both type 2 and IL-22 responses. a, b C57BL/6, Il1rl1-/- and Il22-/- mice were subjected to experimental fungal asthma and administered IL-7 as in Fig. 2. At 24 h after the last organism challenge, the right lungs were collected, homogenized and (a) CCL17 and (b) CCL22 levels were quantified in clarified lung homogenates by ELISA. The Figures illustrates cumulative data from four independent studies. For all graphs, *, ** and *** represent P values of <0.05, <0.01 and <0.001, respectively; n = 4–6 mice/group for each study; each data point/dot represents a single mouse and the line in each group corresponds to the mean
In vivo blockade of the IL-7 receptor CD127 improves some aspects of experimental fungal asthma severity
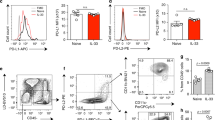
Common γ-chain cytokines are unique in that neutralizing antibodies are ineffective and actually enhance cytokine activity via immune complex formation.22 In fact, we confirmed that neutralizing IL-7 resulted in more severe fungal asthma (data not shown). Therefore, to determine whether targeting IL-7 signaling could affect fungal asthma severity, we employed in vivo blockade of the IL-7 receptor, CD127 (Fig. 5a). The results in Fig. 5 demonstrate that blocking IL-7 signaling in vivo during fungal asthma resulted in a trend towards lower CCL17 (Fig. 5b), but a significant reduction in CCL22 (Fig. 5c) levels. Interesting, blocking IL-7 signaling also negatively affected IL-22 production (Fig. 5d), which correlated with a significant decrease in overall BAL cellularity (Fig. 5e) and, specifically, decreases in CD4 T cells, iNKT cells and eosinophils (Fig. 5f). However, to our surprise, blocking the IL-7 receptor in vivo increased dynamic lung resistance (Fig. 5g). Thus, in vivo blockade of CD127 ameliorated some components of type 2 and IL-22 responses during fungal asthma.
In vivo blockade of the IL-7 receptor CD127 improves some aspects of experimental fungal asthma severity. a C57BL/6 mice were subjected to experimental fungal asthma and on days 7, 9, 11 and 14, mice received rat anti-mouse IL-7Rα/CD127 or rat IgG2a isotype control intraperitoneally. b, c At 24 h after the last organism challenge (b) CCL17 and (c) CCL22 levels were quantified in clarified lung homogenates by ELISA. The Figures illustrates cumulative data from three independent studies. d At 24 h after the last organism challenge, the lungs were lavaged and unfractionated lung lavage cells cultured for 24 h in the presence of A. fumigatus conidia. IL-22 levels were quantified in clarified co-culture supernatants by ELISA. The Figure illustrates cumulative data from two independent studies. e, f At 24 h after the last organism challenge, lung cells were isolated by bronchoalveolar lavage, enumerated, Fc-blocked and stained with a live/dead staining kit followed by staining with fluorochrome-conjugated antibodies corresponding to (e) total BAL cells and (f) CD4 T cells, γδ T cells, iNKT cells and eosinophils. (g) At 24 h after the last organism challenge, dynamic lung resistance was analyzed via mechanical ventilation using the flexiVent system. The Figure illustrates cumulative data from two independent studies. Data are expressed as mean ± SEM. For all graphs, *, ** and *** represent P values of <0.05, <0.01 and <0.001, respectively; n = 4–6 mice/group for each study; each data point/dot represents a single mouse and the line in each group corresponds to the mean
Discussion
The results from the Severe Asthma Research Program (SARP) have led to the most comprehensively characterized cohort of individuals with severe asthma ever assembled. SARP has identified five unique clinical clusters of asthma severity, ranging from mild allergic asthma (cluster 1) to severe fixed airflow asthma (cluster 5).23 A major finding was that a component of each cluster was a high level of atopy, specifically clusters 1, 2 and 4 have a higher degree of atopy/allergy (~80%), whereas clusters 3 and 5 were less atopic (albeit this was still ~65%). Individuals with allergic asthma often have fluctuations in lung function after exposure to an allergen in which they are sensitized.24 Indeed, the highest number of exacerbations per year (at nearly 5) is thought to be in asthmatics categorized as “early onset allergic asthmatics”.24 With respect to environmental allergens, fungi, cockroach allergens and house dust mites are considered the most ubiquitous. Regarding specific fungi, the presence of Penicillium, Aspergillus and Cladosporium prior to the development of asthma symptoms were found to be associated with increased risk of respiratory complications, whereas Alternaria was associated with an increased risk of exacerbation of current asthma.2 Likewise, meta-analysis of studies conducted in the United States on the relationship between dampness and mold/fungi in homes and respiratory health effects revealed a 35–75% increase in reporting respiratory or asthma-related health outcomes.25 Finally, data collected in Europe from eight birth cohorts consisting of >31,000 children found that exposure to fungi and/or dampness during the first 2 years of life was associated with increased risk of the development of asthma.26
We initiated our analysis by examining asthmatics enrolled in SARP who were or were not skin test reactive to Alternaria, Aspergillus or Cladosporium. Collectively, these data showed that asthmatics who are sensitive to fungi demonstrate more obstruction, enhanced responsiveness to bronchodilators and methacholine sensitivity and evidence for increased atopy. As these observations in the fungal (+) cohort may be reflected by differences in immune responsiveness and correlate with immune/inflammatory environments, distinct BAL cytokine profiles may exist. Indeed, previous studies analyzing samples from asthma cohorts have employed bronchoalveolar lavage cytokine phenotyping in an effort to better identify asthma subtypes.27,28 These comparisons are usually made between non-severe and severe asthmatics, often stratified by lung function measurements or between controlled and uncontrolled asthma.29 Although atopic status is often acknowledged, it is usually not a specific focus. From our Luminex analysis, we identified 12 mediators that were elevated in fungal (+) asthmatics. Of these, EGF, IL-1RA and IL-7 demonstrated a significant, negative correlation with the lung function measurement PC20/methacholine challenge test, a well-established method of assessing a key feature of asthma, i.e., airway hyperresponsiveness.30 From these, IL-7 was the most unexpected mediator. While the difference in IL-7 between fungal (+) and fungal (−) asthmatics is small, this was nevertheless an intriguing finding. IL-7 is difficult to detect in many disease systems, as there are many different cell types that express the IL-7 receptor (all T and B lymphocytes, NKTs, gd T cells, ILCs, dendritic cells etc.), and thus the cytokine is constantly depleted. In fact, a recent paper in Immunity examined this in detail and documented cell types that acted as “IL-7 sinks”.17 Therefore, to find any difference in IL-7 was interesting. Very little is known regarding IL-7 and human asthma. Gene linkage studies on chromosome 5p13 have previously identified four genes associated with asthma in different populations, one of which was IL-7R.31 Another study in humans has shown that segmental allergen challenge with cat allergen, ragweed or house dust mite elicited IL-7, which correlated with eosinophil numbers in BAL fluid as well as eosinophil activation.32 Likewise, comparing IL-7 levels in BAL fluid from healthy controls vs. asthmatics observed increased IL-7 in the latter.29 Differences between our study and these include IL-7 detection methods (enzyme-linked immunosorbent assay (ELISA), different Luminex kits), BAL volume and fluid processing and normalization.
Replicating the elevated levels observed in human fungal (+) asthmatics, our data demonstrated that IL-7 administration during experimental fungal asthma dramatically worsened lung function, increased proallergic, type 2 and proinflammatory responses and augmented the recruitment of both CD4 T cells and eosinophils. Although the dose of IL-7 employed in our studies was based on published studies,33,34 we recognize that this dose (and those in other published studies) may be at “super-physiologic” levels, and thus is a caveat in our study. In line with our observations, mice deficient in IL-7R acutely15 or chronically35 exposed to an extract from asthma-associated fungus Alternaria have reduced eosinophils in the lung. A caveat here is that multiple T lymphocyte and innate-like lymphocyte populations are developmentally compromised in the absence of IL-7 signaling, and therefore development of allergic responses would be expected to be hampered. Indeed, we have examined our experimental fungal asthma model in Il7-/- mice and observed significantly reduced proallergic and inflammatory mediator levels (data not shown). An additional response that was significantly affected by elevating the levels of IL-7 was IL-22. We have previously published that mice deficient in IL-22 have less severe fungal asthma and that neutralizing IL-22 in normal mice improved lung function.12 IL-7-mediated IL-22 production provided us the opportunity to identify cells that contribute to immunopathogenesis during fungal asthma putatively via IL-22 production. To this end, employing IL-22 reporter mice resulted in the identification of iNKT cells, CD4 T cells, γδ T cells and ILC3s as lung cell sources of IL-22 during experimental fungal asthma. Studies have shown that γδ T-cell homeostasis requires IL-736 as do certain iNKT cell populations.37 In fact, a recent study reported that IL-17+RORγt+iNKT cells in the spleen depend exclusively on IL-7 for homeostasis and survival.38 In turn, IL-7-treated IL-22 reporter mice demonstrated a significant increase in IL-22-producing iNKT cells, while the levels of IL-22-producing γδ T cells remained relatively unchanged. Likewise, normal mice treated with IL-7 demonstrated expansion in the total iNKT cell population but not the total γδ T-cell population. The presence of and a role for iNKT cells in human asthma is controversial, with studies arguing that iNKT cells are39,40 and are not41,42 elevated. These studies however are difficult to compare as staining procedures, investigations of different iNKT cell subsets (CD4+, CD8+ etc.) and specimen source (sputum, lung lavage fluid, peripheral blood) are common variables. Nevertheless, higher Vα24+ and 6B11+ iNKT cells have been observed in sputum from asthmatics with high eosinophil counts43 as well as in pediatric asthmatics during asthma exacerbation.44 Moreover, numerous experimental studies indicate an immunopathogenic role for iNKT cells,45,46 including a recent study showing that a fungal glycosphingolipid contributes to airway hyperreactivity via iNKT cell activation and type 2 cytokine production.47
Although we observed iNKT cells producing IL-22 during fungal asthma, they may also act as a source of type 2 cytokines as well. In fact, we have observed iNKT cells producing IL-4 during experimental fungal asthma using the 4get IL-4 reporter mice48 (data not shown). As IL-7 increased type 2 as well as IL-22 responses and also increased iNKT cell numbers, we questioned whether IL-7-mediated immunopathogenesis required one response over the other. Employing CCL17 and CCL22 as biomarkers of severity, we found that IL-7 administration generally resulted in an increase in these mediators even in the absence of the IL-33 signaling or IL-22 signaling. Although some reports suggest that IL-33 may not be involved in the generation of type 2 responses during allergic responses,49,50 other reports (including in fungal asthma) suggest a required role for IL-33 in type 2 responses.51,52,53 Collectively, these data suggest that IL-7 requires both type 2 and IL-22 responses, in that the absence of one response is not sufficient to eliminate the immunopathogenic effects of IL-7.
Like most common γ-chain cytokines, IL-7 is broadly considered to be essential for lymphocyte development and this cytokine family is becoming newly appreciated for activities on innate lymphoid cell populations.54,55 To this end, increases in common γ-chain cytokines may result in increased or sustained innate lymphoid cell survival and/or activation leading to immunopathogenic responses during allergic asthma. Therefore, can common γ-chain cytokines, specifically IL-7, be a therapeutic target? A previous clinical study evaluating daclizumab, a humanized IgG1 monoclonal antibody against the IL-2R alpha chain (CD25), was reported to improve FEV1, reduce daytime asthma symptoms and prolong time to exacerbation.56 To this end, we found that blocking the IL-7R alpha chain resulted in lower proallergic chemokine levels as well as lower IL-22 production. Although TSLP also utilizes the IL-7R alpha chain,57 we feel our observations were not a result of impacting TSLP signaling, as we have previously reported that TSLP is not induced in our experimental fungal asthma model.12 To our surprise however, blocking the IL-7R alpha receptor during experimental fungal asthma resulted in a small but significant increase in dynamic lung resistance, indicating that this treatment essentially worsened lung function. Although this finding is opposite to our hypothesis, it is nonetheless a compelling observation. Previous studies blocking IL-7R alpha receptor in vivo in an autoimmune type 1 diabetes model demonstrated attenuated Th17 generation, but no effect on disease severity.58 In contrast, other studies have demonstrated the opposite effect of IL-7R blockade in experimental diabetes, showing increased PD-1 expression on effector/memory T cells (that rendered them less immunopathogenic)59 as well as increasing the numbers of Tregs60 as putative mechanisms of decreased disease severity. However, other studies suggest that IL-7 is required for maintaining memory Tregs in the skin61 as well as memory T cells.62 Likewise, another study demonstrated that blocking IL-7 receptor in vivo may reduce Tregs in the spleens of young, but not old, mice.63 As these mechanisms may or may not explain worse lung function in anti-CD127-treated mice during fungal asthma, a goal in future studies will be to determine what proallergic and inflammatory responses are modulated by anti-CD127 treatment during fungal asthma.
In conclusion, fungal sensitivity in human asthma is associated with more severe disease. A correlate of severity is elevated levels of the common γ-chain cytokine IL-7, which experimentally promotes increased CD4 T cell, iNKT cell and eosinophil accumulation in the lung leading to heightened type 2 and IL-22 responses, both of which are required for the full effects of IL-7-mediated fungal asthma severity. Therapeutic blockade of IL-7 signaling in vivo improved some immunologic aspects of experimental fungal asthma severity, but slightly worsened physiologic aspects. Our studies identify the common γ-chain cytokine family as potential new targets in allergic asthma.
Materials and methods
Subjects
Patients with mild to severe asthma were comprehensively characterized according to the NHLBI (National Heart, Lung, and Blood Institute) SARP phenotype protocol at Wake Forest School of Medicine as previously described.64 Briefly, non-smoking subjects (<5 pack years) who met American Thoracic Society (ATS) criteria for the diagnosis of asthma (enriched for severe asthma) provided informed consent (Wake Forest Institutional Review Board approved protocol #IRB00021507). Evaluation included spirometry, bronchodilator reversibility and bronchial responsiveness to methacholine (BHR), total serum IgE level, exhaled NO measurement, bronchoalveolar lavage and administration of questionnaires that characterized asthma symptoms, quality of life, medications and healthcare utilization.64 Fungal sensitivity was identified via skin prick test. Subjects withheld over-the-counter and first- or second-generation antihistamines, H2 antagonists and antidepressants for 3 days prior to skin tests. Those taking beta-2 blockers for comorbidities did not undergo skin prick tests. Tests to 12 allergens (eastern tree Mix, Aspergillus mix, southern grass mix, ragweed short AgE, cockroach mix, Alternaria tenuis, Cladosporium herbarum, dog epithelia/mixed breeds, standardized cat hair and standardized Dermatophagoides farinae and Dermatophagoides pteronyssinus), diluent and histamine were primarily performed with Multitest II (Lincoln Diagnostics) by certified SARP coordinator or technician. Tests were read after 15 min and largest and perpendicular wheal diameters recorded and measured on transparent tape. A positive response was recorded for a wheal with largest diameter of 3 mm or greater than diluent (negative control). The largest and perpendicular wheal diameters were used to calculate the elliptical area of the wheal reaction; the reaction area for diluent, if any, was subtracted.
Bronchoalveolar lavage, processing and Luminex® analysis
Investigative bronchoscopy was performed on a subset of SARP subjects with all levels of asthma severity.64 Three to four aliquots of 50 ml of normal saline (total of 150–200 ml, prewarmed to 37 °C) were instilled into a lung segment and recovered with gentle hand suction by syringe. The recovered BAL fluid from all aliquots is measured (for volume of recovery), combined as a single sample, and retained on ice during processing. The BAL volume recovered is centrifuged in 50 ml centrifuge tubes for 10 min at 450 × g at 4 °C. BALF supernatants are combined and 15 ml aliquots placed into Millipore ultrafree concentrator (5000 MW cutoff) and centrifuged at 2000 × g for 30 min with additional time to concentrate 20× (0.75 ml). All concentrates from individual concentrators are combined to prepare a single, homogeneous concentrate which was distributed in appropriate volumes (250 or 500 µl) and stored frozen at −80 °C. All subjects undergoing bronchoscopy were self-selected for this portion of the SARP study. Biospecimens were randomly selected without a priori selection based on asthma severity, based on whether they were skin test positive or negative for Alternaria, Aspergillus or Cladosporium. Cell-free supernatants were aliquoted and stored at −80 °C before use in Luminex® analyses. Supernatants were assayed for different inflammatory cytokine, chemokine and growth factor protein concentrations using Milliplex® Human Cytokine/Chemokine Panels I, II and III (catalog numbers HCYTOMAG-60K-PX41, HCYP2MAG-62K-PX23 and HCYP3MAG-63K, respectively, EMD Millipore). Standards for determination of linear curve plus two control samples representing high and low levels of each cytokine/chemokine were included in each assay. The protein content of the BAL fluid supernatant was assessed by using a Coomassie (Bradford) protein assay (Pierce Biotechnology, Rockford, Ill) read at an absorbance of 595 nm with a detection limit 1 μg/ml. Samples were analyzed under University of Alabama at Birmingham (UAB) Institutional Review Board-approved protocol # X130827009.
Mice
WT BL/6 mice, 6 to 8 weeks of age, were obtained from Taconic (Hudson, NY). Il1rl1-/- (ST2/IL-33R) mice were a kind gift from Dr. Andrew McKenzie, Cambridge University. Il22-/- mice were employed as previously described.12 IL22Cre was purchased from Jackson Laboratory and crossed to R26ReYFP (expressing eYFP from the Rosa26 promoter) generating IL22CreR26ReYFP reporter mice as previously described.65 All animals were housed in a specific pathogen-free, Association for Assessment and Accreditation of Laboratory Animal Care-certified facility and handled according to Public Health Service Office of Laboratory Animal Welfare policies after review by the UAB Institutional Animal Care and Use Committee.
Experimental fungal asthma model
A. fumigatus isolate 13073 (ATCC, Manassas, VA) was maintained on potato dextrose agar for 5–7 days at 37 °C. Conidia were harvested by washing the culture flask with 50 ml of sterile phosphate-buffered saline (PBS) supplemented with 0.1% Tween-20. The conidia were then passed through a sterile 40 μm nylon membrane to remove hyphal fragments and enumerated on a hemacytometer. The repeated A. fumigatus exposure model was employed as previously described.12 Briefly, mice were lightly anesthetized with isoflurane and administered 1 × 107 live A. fumigatus conidia in a volume of 50 μl of PBS intratracheally (i.t.). Starting at day 7, mice were challenged i.t. with 1 × 106 conidia live A. fumigatus in 50 μl of PBS daily for 5 days, rested for 2 days, and challenged daily for another 3 days. Mice undergoing a control/sham protocol were administered the first challenge of 1 × 107 A. fumigatus conidia as described above. Starting at day 7, they received 50 μl of PBS for 5 days, rested for 2 days and then daily 50 μl of PBS for the remaining 3 days. At 24 h after the final challenge, immune and physiologic measures were assessed as described below.
Analysis of IL-22 cell sources
WT BL/6 and IL22CreR26ReYFP reporter mice were subjected to fungal asthma. At 24 h after the last challenge, lungs were collected and minced in Iscove's modified Dulbecco's medium (Sigma, St. Louis, MO) supplemented with 1% penicillin–streptomycin–glutamine (Mediatech, Herndon, VA), 10% heat-inactivated fetal bovine serum (Invitrogen, Carlsbad, CA) and 0.4 mg/ml polymyxin B (Thermo Fisher), followed by incubation for 60 min with tissue culture-grade type IV collagenase (1 mg/ml; Sigma, St. Louis, MO) in a 37 °C orbital shaker at 100 rpm. The cell suspension was filtered through sterile 70 μm nylon filters, red blood cells lysed with ACK buffer (Lonza, Walkersville, MD), then filtered through a sterile 40 μm nylon filters to create single-cell preparations. Single-cell suspensions were cultured in the presence of IL-1β alone (10 ng/ml) for 4 h. Markers for specific cell types are presented in Fig. 3 and Supplemental Fig. 2.
Analysis of IL-22 production in vitro
Mice were chronically exposed to A. fumigatus as described. At 24 h after the last challenge, the right lung was collected and single-cell suspensions isolated as above. One million cells in a volume of 200 μl were cultured for 24 h in the presence of A. fumigatus conidia in a 1:1 ratio followed by assessment of IL-22 by ELISA. In some studies, lung cells were collected from bronchoalveolar lavage fluid and cultured in a volume of 200 μl for 24 h (1 × 105) in the presence of A. fumigatus conidia in a 1:1 ratio
In vivo IL-7 treatment and IL-7 receptor (CD127) blockade
For in vivo IL-7 treatment, WT BL/6 mice were chronically exposed to A. fumigatus as described. On days 7, 9, 11 and 14, mice received 1.5 µg of carrier-free recombinant murine IL-7 (R&D Systems) (dose based on refs. 33,34) in a volume of 50 µl intratracheally. Controls received 50 µl of diluent (PBS) intratracheally. For in vivo anti-IL-7R (CD127) blockade, WT BL/6 mice were chronically exposed to A. fumigatus as described. On days 7, 9, 11 and 14, mice received 0.5 mg of InVivoMAb rat anti-mouse IL-7Rα/CD127 (Catalog #BE0065, Bio X Cell, West Lebanon, NH) in a volume of 0.2 ml intraperitoneally. Controls received 0.5 mg of InVivoMAb Rat IgG2a Isotype control (anti Trinitrophenol; Catalog #BE0089, Bio X Cell).
Cytokine and chemokine assessment
The right lung was homogenized in PBS supplemented with Complete Mini protease inhibitor tablets (Roche), clarified by centrifugation and stored at −80 °C. Supernatants from lung homogenates were analyzed for protein levels of 32 cytokines and chemokines using a Milliplex multiplex suspension cytokine array (Millipore) according to the manufacturer’s instructions. The data were analyzed using Bio-Plex Manager software (Bio-Rad Laboratories). IL-33, CCL17 and CCL22 levels were quantified by ELISA (R&D Systems). In the studies examining the effects of IL-7R blockade, proallergic chemokine levels were analyzed in bronchoalveolar lavage.
Flow cytometric analysis
Lung cells were isolated via bronchoalveolar lavage as previously described.66 Cells were washed and Fc receptors were blocked with Mouse BD Fc Block™ (BD Biosciences, San Diego, CA) at 4 °C for 20 min. Thereafter, cells were stained with a single-color LIVE/DEAD® Fixable Dead Cell Stain (Invitrogen) followed by labeling with specific immune cell surface markers. The following staining parameters were employed: eosinophils as CD11b+Siglec F+Ly6G and Ly-6Clo/neg, neutrophils as CD11b+Ly6G+(1A8), T cells as CD3+CD4+, NKT cells as TCR-beta+, murine CD1d tetramer+ and γδ T cells as TCR-delta+ and CD3+ (all antibodies purchased from eBiosciences and BD Biosciences). Samples were acquired using a four laser, 20-parameter analytic BD™ LSR II and data were analyzed using FlowJo software (Tree Star, Ashland, OR). Unstained cells served as a control for background fluorescence and gating. Samples were acquired using BD™ LSR II cytometer (BD Biosciences) and data were analyzed using FlowJo software (Tree Star, Ashland, OR).
Pulmonary function assessment
A tracheostomy was performed on individual anesthetized A. fumigatus-exposed mice. Each animal was then attached to a computer controlled volume ventilator (flexiVent, SCIREQ Montreal, PQ, Canada). Regular breathing was set at 150 bpm, with volume and pressure controlled by the flexiVent system based on individual animal weights. Positive end-expiratory pressure was set to 2 cm H2O and measured during each breath stroke. Respiratory input impedance (Zrs) was measured using the Forced Oscillation Technique controlled by the flexiVent system. The Single-Compartment Model was used to describe dynamic lung resistance. All measurements were collected at baseline and after a linear dose response with methacholine challenge (10–40 mg/ml) as previously described.67
Statistics
Data were analyzed using GraphPad Prism, version 5.0, statistical software (GraphPad Software, San Diego, CA). Comparisons between groups when data were normally distributed were made with the two-tailed unpaired Student t test. Significance was accepted at a P value of < 0.05.
References
Denning, D. W., O’Driscoll, B. R., Hogaboam, C. M., Bowyer, P. & Niven, R. M. The link between fungi and severe asthma: a summary of the evidence. Eur. Respir. J. 27, 615–626 (2006).
Sharpe, R. A., Bearman, N., Thornton, C. R., Husk, K. & Osborne, N. J. Indoor fungal diversity and asthma: a meta-analysis and systematic review of risk factors. J. Allergy Clin. Immunol. 135, 110–122 (2015).
Agarwal, R. Severe asthma with fungal sensitization. Curr. Allergy Asthma Rep. 11, 403–413 (2011).
Denning, D. W. et al. Fungal allergy in asthma-state of the art and research needs. Clin. Transl. Allergy 4, 14 (2014).
O’Driscoll, B. R., Hopkinson, L. C. & Denning, D. W. Mold sensitization is common amongst patients with severe asthma requiring multiple hospital admissions. BMC Pulm. Med. 5, 4 (2005).
O’Driscoll, B. R. et al. Comparison of skin prick tests with specific serum immunoglobulin E in the diagnosis of fungal sensitization in patients with severe asthma. Clin. Exp. Allergy 39, 1677–1683 (2009).
Fairs, A. et al. IgE sensitization to Aspergillus fumigatus is associated with reduced lung function in asthma. Am. J. Respir. Crit. Care Med. 182, 1362–1368 (2010).
Maurya, V., Gugnani, H. C., Sarma, P. U., Madan, T. & Shah, A. Sensitization to Aspergillus antigens and occurrence of allergic bronchopulmonary aspergillosis in patients with asthma. Chest 127, 1252–1259 (2005).
Burgel, P. R. et al. High prevalence of azole-resistant Aspergillus fumigatus in adults with cystic fibrosis exposed to itraconazole. Antimicrob. Agents Chemother. 56, 869–874 (2012).
Kraemer, R., Delosea, N., Ballinari, P., Gallati, S. & Crameri, R. Effect of allergic bronchopulmonary aspergillosis on lung function in children with cystic fibrosis. Am. J. Respir. Crit. Care Med. 174, 1211–1220 (2006).
Bafadhel, M. et al. Aspergillus fumigatus during stable state and exacerbations of COPD. Eur. Respir. J. 43, 64–71 (2013).
Lilly, L. M. et al. The beta-glucan receptor Dectin-1 promotes lung immunopathology during fungal allergy via IL-22. J. Immunol. 189, 3653–3660 (2012).
Zenewicz, L. A. & Flavell, R. A. Recent advances in IL-22 biology. Int. Immunol. 23, 159–163 (2011).
Nair, P. et al. Airway hyperresponsiveness in asthma: measurement and clinical relevance. J. Allergy Clin. Immunol. Pract. 5, 649–659 (2017). e642.
Doherty, T. A. et al. STAT6 regulates natural helper cell proliferation during lung inflammation initiated by Alternaria. Am. J. Physiol. Lung. Cell Mol. Physiol. 303, L577–L588 (2012).
Gao, F. S. et al. Effects of chronic exposure to Aspergillus fumigatus on epidermal growth factor receptor expression in the airway epithelial cells of asthmatic rats. Exp. Lung Res. 40, 298–307 (2014).
Martin, C. E. et al. Interleukin-7 availability is maintained by a hematopoietic cytokine sink comprising innate lymphoid cells and T cells. Immunity 47, 171–182 (2017). e174.
Ramaprakash, H. et al. Targeting ST2L potentiates CpG-mediated therapeutic effects in a chronic fungal asthma model. Am. J. Pathol. 179, 104–115 (2011).
Hartl, D. et al. Chemokines indicate allergic bronchopulmonary aspergillosis in patients with cystic fibrosis. Am. J. Respir. Crit. Care Med. 173, 1370–1376 (2006).
Latzin, P. et al. Comparison of serum markers for allergic bronchopulmonary aspergillosis in cystic fibrosis. Eur. Respir. J. 31, 36–42 (2008).
Schuh, J. M. et al. Airway hyperresponsiveness, but not airway remodeling, is attenuated during chronic pulmonary allergic responses to Aspergillus in CCR4-/- mice. FASEB J. 16, 1313–1315 (2002).
Votavova, P., Tomala, J. & Kovar, M. Increasing the biological activity of IL-2 and IL-15 through complexing with anti-IL-2 mAbs and IL-15Ralpha-Fc chimera. Immunol. Lett. 159, 1–10 (2014).
Jarjour, N. N. et al. Severe asthma: lessons learned from the National Heart, Lung, and Blood Institute Severe Asthma Research Program. Am. J. Respir. Crit. Care Med. 185, 356–362 (2012).
McDonald, V. M. & Gibson, P. G. Exacerbations of severe asthma. Clin. Exp. Allergy 42, 670–677 (2012).
Fisk, W. J., Lei-Gomez, Q. & Mendell, M. J. Meta-analyses of the associations of respiratory health effects with dampness and mold in homes. Indoor Air. 17, 284–296 (2007).
Tischer, C. G. et al. Meta-analysis of mould and dampness exposure on asthma and allergy in eight European birth cohorts: an ENRIECO initiative. Allergy 66, 1570–1579 (2011).
Bhavnani, S. K. et al. How cytokines co-occur across asthma patients: from bipartite network analysis to a molecular-based classification. J. Biomed. Inform. 44(Suppl 1), S24–S30 (2011).
Brzozowska, A. et al. Exhaled nitric oxide correlates with IL-2, MCP-1, PDGF-BB and TIMP-2 in exhaled breath condensate of children with refractory asthma. Post. Dermatol. Alergol. 32, 107–113 (2015).
Hosoki, K. et al. Analysis of a panel of 48 cytokines in BAL fluids specifically identifies IL-8 levels as the only cytokine that distinguishes controlled asthma from uncontrolled asthma, and correlates inversely with FEV1. PLoS One 10, e0126035 (2015).
El-Gammal, A. I. et al. Comparison of the provocative concentration of methacholine causing a 20% fall in FEV1 between the AeroEclipse II breath-actuated nebulizer and the wright nebulizer in adult subjects with asthma. Ann. Am. Thorac. Soc. 12, 1039–1043 (2015).
Kurz, T. et al. Fine mapping and positional candidate studies on chromosome 5p13 identify multiple asthma susceptibility loci. J. Allergy Clin. Immunol. 118, 396–402 (2006).
Kelly, E. A. et al. Potential contribution of IL-7 to allergen-induced eosinophilic airway inflammation in asthma. J. Immunol. 182, 1404–1410 (2009).
Shindo, Y. et al. Interleukin 7 immunotherapy improves host immunity and survival in a two-hit model of Pseudomonas aeruginosa pneumonia. J. Leukoc. Biol. 101, 543–554 (2017).
Ruan, S., Samuelson, D. R., Assouline, B., Morre, M. & Shellito, J. E. Treatment with interleukin-7 restores host defense against pneumocystis in CD4+T-lymphocyte-depleted mice. Infect. Immun. 84, 108–119 (2015).
Doherty, T. A. et al. Lung type 2 innate lymphoid cells express cysteinyl leukotriene receptor 1, which regulates TH2 cytokine production. J. Allergy Clin. Immunol. 132, 205–213 (2013).
Baccala, R. et al. Gamma delta T cell homeostasis is controlled by IL-7 and IL-15 together with subset-specific factors. J. Immunol. 174, 4606–4612 (2005).
Liang, B. et al. Role of hepatocyte-derived IL-7 in maintenance of intrahepatic NKT cells and T cells and development of B cells in fetal liver. J. Immunol. 189, 4444–4450 (2012).
Webster, K. E. et al. IL-17-producing NKT cells depend exclusively on IL-7 for homeostasis and survival. Mucosal Immunol. 7, 1058–1067 (2014).
Akbari, O. et al. CD4+invariant T-cell-receptor+natural killer T cells in bronchial asthma. N. Engl. J. Med. 354, 1117–1129 (2006).
Matangkasombut, P. et al. Natural killer T cells in the lungs of patients with asthma. J. Allergy Clin. Immunol. 123, 1181–1185 (2009).
Rock, M. et al. Effect of allergen challenge on the percentage of natural killer T cells in patients with atopic asthma. Ann. Allergy Asthma Immunol. 102, 432–437 (2009).
Brooks, C. R., Weinkove, R., Hermans, I. F., Van Dalen, C. J. & Douwes, J. Invariant natural killer T cells and asthma: immunologic reality or methodologic artifact? J. Allergy Clin. Immunol. 126, 882–885 (2010).
Koh, Y. I. & Shim, J. U. Association between sputum natural killer T cells and eosinophilic airway inflammation in human asthma. Int. Arch. Allergy Immunol. 153, 239–248 (2010).
Pham-Thi, N. et al. Enhanced frequency of immunoregulatory invariant natural killer T cells in the airways of children with asthma. J. Allergy Clin. Immunol. 117, 217–218 (2006).
Lisbonne, M. et al. Cutting edge: invariant V alpha 14 NKT cells are required for allergen-induced airway inflammation and hyperreactivity in an experimental asthma model. J. Immunol. 171, 1637–1641 (2003).
Meyer, E. H. et al. Glycolipid activation of invariant T cell receptor+NK T cells is sufficient to induce airway hyperreactivity independent of conventional CD4+T cells. Proc. Natl. Acad. Sci. USA 103, 2782–2787 (2006).
Albacker, L. A. et al. Invariant natural killer T cells recognize a fungal glycosphingolipid that can induce airway hyperreactivity. Nat. Med. 19, 1297–1304 (2013).
Mohrs, K., Wakil, A. E., Killeen, N., Locksley, R. M. & Mohrs, M. A two-step process for cytokine production revealed by IL-4 dual-reporter mice. Immunity 23, 419–429 (2005).
Hoshino, K. et al. The absence of interleukin 1 receptor-related T1/ST2 does not affect T helper cell type 2 development and its effector function. J. Exp. Med. 190, 1541–1548 (1999).
Vannella, K. M. et al. Combinatorial targeting of TSLP, IL-25, and IL-33 in type 2 cytokine-driven inflammation and fibrosis. Sci. Transl. Med 8, 337ra365 (2016).
Castanhinha, S. et al. Pediatric severe asthma with fungal sensitization is mediated by steroid-resistant IL-33. J. Allergy Clin. Immunol. 136, 312–322 (2015). e317.
Sjoberg, L. C. et al. Interleukin 33 exacerbates antigen driven airway hyperresponsiveness, inflammation and remodeling in a mouse model of asthma. Sci. Rep. 7, 4219 (2017).
Jackson, D. J. et al. IL-33-dependent type 2 inflammation during rhinovirus-induced asthma exacerbations in vivo. Am. J. Respir. Crit. Care Med. 190, 1373–1382 (2014).
Roediger, B. et al. IL-2 is a critical regulator of group 2 innate lymphoid cell function during pulmonary inflammation. J. Allergy Clin. Immunol. 136, 1653–1663 (2015).
Turner, J. E. et al. IL-9-mediated survival of type 2 innate lymphoid cells promotes damage control in helminth-induced lung inflammation. J. Exp. Med. 210, 2951–2965 (2013).
Busse, W. W. et al. Daclizumab improves asthma control in patients with moderate to severe persistent asthma: a randomized, controlled trial. Am. J. Respir. Crit. Care Med. 178, 1002–1008 (2008).
Park, L. S. et al. Cloning of the murine thymic stromal lymphopoietin (TSLP) receptor: formation of a functional heteromeric complex requires interleukin 7 receptor. J. Exp. Med. 192, 659–670 (2000).
Devarajan, P., Miska, J., Lui, J. B., Swieboda, D. & Chen, Z. Opposing effects of CTLA4 insufficiency on regulatory versus conventional T cells in autoimmunity converge on effector memory in target tissue. J. Immunol. 193, 4368–4380 (2014).
Penaranda, C. et al. IL-7 receptor blockade reverses autoimmune diabetes by promoting inhibition of effector/memory T cells. Proc. Natl. Acad. Sci. USA 109, 12668–12673 (2012).
Lee, L. F. et al. Anti-IL-7 receptor-alpha reverses established type 1 diabetes in nonobese diabetic mice by modulating effector T-cell function. Proc. Natl. Acad. Sci. USA 109, 12674–12679 (2012).
Gratz, I. K. et al. Cutting Edge: memory regulatory t cells require IL-7 and not IL-2 for their maintenance in peripheral tissues. J. Immunol. 190, 4483–4487 (2013).
McKinstry, K. K. et al. Effector CD4 T-cell transition to memory requires late cognate interactions that induce autocrine IL-2. Nat. Commun. 5, 5377 (2014).
Chougnet, C. A. et al. A major role for Bim in regulatory T cell homeostasis. J. Immunol. 186, 156–163 (2011).
Moore, W. C. et al. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am. J. Respir. Crit. Care Med. 181, 315–323 (2010).
Ahlfors, H. et al. IL-22 fate reporter reveals origin and control of IL-22 production in homeostasis and infection. J. Immunol. 193, 4602–4613 (2014).
Nelson, M. P., Metz, A. E., Li, S., Lowell, C. A. & Steele, C. The absence of Hck, Fgr and Lyn tyrosine kinases augments lung innate immune responses to Pneumocystis murina. Infect. Immun. 77, 1790–1797 (2009).
Werner, J. et al. Requisite role for the Dectin-1 beta-glucan receptor in pulmonary defense against Aspergillus fumigatus. J. Immunol. 182, 4938–4946 (2009).
Dudakov, J. A., Hanash, A. M. & van den Brink, M. R. Interleukin-22: immunobiology and pathology. Annu. Rev. Immunol. 33, 747–785 (2015).
Acknowledgements
This work was supported by PHS grants HL109164 (to D.A.M.) and HL136211 and HL122426 (to C.S.).
Author information
Authors and Affiliations
Contributions
D.A.M., K.M.R. and C.S. conceived of the study. A.T.H., E.J.A. and D.A.M. conducted the human subjects clinical data and sample acquisition and analysis with the exception of the cytokine levels in lung lavage fluid (C.W.D., J.P.B. and C.S.). K.M.R. performed all experiments and interpreted the data along with C.S. K.M.R. and C.S. wrote the manuscript. D.A.M. and C.S. generated the funds to perform the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Reeder, K.M., Dunaway, C.W., Blackburn, J.P. et al. The common γ-chain cytokine IL-7 promotes immunopathogenesis during fungal asthma. Mucosal Immunol 11, 1352–1362 (2018). https://doi.org/10.1038/s41385-018-0028-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41385-018-0028-1
This article is cited by
-
γδ T Lymphocytes in Asthma: a Complicated Picture
Archivum Immunologiae et Therapiae Experimentalis (2021)
-
Interleukin-7 protects against bacterial respiratory infection by promoting IL-17A-producing innate T-cell response
Mucosal Immunology (2020)