Abstract
Upon oral infection with Toxoplasma gondii cysts (76 K strain) tachyzoites are released into the intestinal lumen and cross the epithelial barrier causing damage and acute intestinal inflammation in C57BL/6 (B6) mice. Here we investigated the role of microbiota and IL-22 in T.gondii-induced small intestinal inflammation. Oral T.gondii infection in B6 mice causes inflammation with IFNγ and IL-22 production. In IL-22-deficient mice, T.gondii infection augments the Th1 driven inflammation. Deficiency in either IL-22bp, the soluble IL-22 receptor or Reg3γ, an IL-22-dependent antimicrobial lectin/peptide, did not reduce inflammation. Under germ-free conditions, T.gondii-induced inflammation was reduced in correlation with parasite load. But intestinal inflammation is still present in germ-free mice, at low level, in the lamina propria, independently of IL-22 expression. Exacerbated intestinal inflammation driven by absence of IL-22 appears to be independent of IL-22 deficiency associated-dysbiosis as similar inflammation was observed after fecal transplantation of IL-22-/- or WT microbiota to germ-free-WT mice. Our results suggest cooperation between parasite and intestinal microbiota in small intestine inflammation development and endogenous IL-22 seems to exert a protective role independently of its effect on the microbiota. In conclusion, IL-22 participates in T.gondii induced acute small intestinal inflammation independently of microbiota and Reg3γ.
Similar content being viewed by others
Introduction
Inflammatory bowel diseases (IBD) are multifactorial with increasing prevalence resulting from environmental factors, genetic susceptibility, microbiota disturbance and immune dysregulation.1 Crohn disease (CD), a type of IBD, may affect any part of the gastro-intestinal tract in contrast to ulcerative colitis which is restricted to the colon. Ileal CD immunopathology affects a majority of patients and can be mimicked in part by Toxoplasma gondii (T.gondii) infection administered by the oral route in C57BL/6 inbred mice (B6).2,3,4 Oral infection with T.gondii cysts causes an acute lethal small intestine inflammation in B6 mice which is characterized by epithelial barrier disruption, neutrophil recruitment, activation of macrophages and inflammation.5,6
Inflammatory cytokines as Th17 cytokines including IL-22 are upregulated in IBD and IL-22 is expressed in inflamed colonic lesions and serum of patients with CD.7,8,9 IL-22 expression is also increased in experimental colitis induced using Dextran Sodium Sulfate (DSS) in drinking water and in CD45RBhi transfer model.10 The inflammation is exacerbated in IL-22 deficient mice or after treatment by anti-IL-22 antibody in DSS-induced colitis,10,11 illustrating the protective role of endogenous IL-22 into the colonic mucosa. IL-22 has also been shown to be required for appropriate response to the natural mouse intestinal pathogen Citrobacter rodentium.12,13,14
By contrast, a pathogenic role for IL-22 was identified in T.gondii intestinal inflammation induced by oral infection with 50–100 cysts of ME49 strain.15,16 However the pathogenicity depends on the strain of T.gondii used, the route of infection and the dose administered.6
IL-22 is a cytokine of the IL-10 family that signals through heterodimeric receptors composed of IL-22Ra1, its specific subunit, and IL-10R2, a chain common to several IL-10 family members.17,18 IL-22 fixation to the membrane IL-22Ra1/IL-10R2 receptor, results in JAK/STAT signaling pathway activation.19 A soluble receptor, the IL-22 binding protein (IL-22BP or IL-22Ra2) binds IL-22 with higher affinity than the membrane heterodimeric receptor20 and acts as an antagonist by neutralizing IL-22 activity.21,22,23 During DSS-induced colitis, IL-22BP was down-regulated, enhancing IL-22 bioactivity and a tumor promoting effect in IL-22BP KO mice.24 Further, IL-22BP antagonized the protective effect of IL-22 during DSS colitis in rats,25 suggesting that the role of the soluble receptor in intestinal inflammation is still not yet fully understood.
IL-22 plays a key role in bacterial defense. Indeed, IL-22 stimulates the expression of mucin, that prevents the physical penetration of bacteria, MUC1, −3, −10, and −13 by colon epithelial cells in a STAT3-dependent manner.26 Further, IL-22 confers protection via the STAT3 signalling pathway by inducing the production of antimicrobial peptides such as BD-2, BD-3,27 S100A7-9,28,29 lipocalin-2 (LCN2)30 and Reg3β/γ.12 Reg3 proteins, which belong to the family of secreted C-type lectins, have been reported to kill some Gram-positive bacteria by interacting with peptidoglycan carbohydrate,31,32 and are critical for intestinal cell growth33 and microbiota localization in the small intestine.34 Dysbiosis observed in IL-22-deficient mice was shown to be associated with altered Reg3β and Reg3γ expression35 reinforcing the concept that IL-22 is important to maintain the intestinal microbiota via antimicrobial peptide production.
Qualitative and/or quantitative studies of changes of the intestinal microbiota revealed a dysbiosis upon T.gondii-induced intestinal inflammation characterized by a shift of bacterial composition to a majority of Enterobacteriaceae from Proteobacteria phylum and a reduced number of Bacteroidetes.36,37,38,39,40,41 Moreover, antibiotics treated mice showed a reduced T.gondii-induced small intestinal inflammation whereas re-colonization of these mice with defined Gram-negative species such as E.coli or Bacteroides/Prevotella causes increased intestinal inflammation after T.gondii infection.42 Similarly, emergence of colitogenic bacteria belonging to E.coli species has also been detected in the ileal mucosa of patients with IBD.43
Despite the fact that dysbiosis was well documented in response to T.gondii-infection, the role of IL-22 in microbiota maintenance remains to be addressed in this model of small intestinal inflammation. It is essential to understand by which mechanisms IL-22 can modulate intestinal immunopathology. Thus, here we report a protective role of endogenous IL-22 in small intestinal inflammation induced by oral infection with 35 cysts of T.gondii type II strain 76 K. We excluded the hypothesis that exacerbated inflammation in IL-22-/- mice is linked to dysbiosis due to IL-22-deficiency or to an unpaired antimicrobial peptide production.
Results
High susceptibility of IL-22-deficient mice to T. gondii-induced small intestinal inflammation
T.gondii induces epithelial damage associated with neutrophil recruitment and Th1 inflammation. Il22 and Il22bp expression were examined over 8 days after T.gondii infection in the small intestinal mucosa (Fig. 1a). Il22 messengers increase at 6 days post infection reaching a maximum at 7 days and progressing toward resolution at 8 days, while Il22bp expression is reduced on day 5–8 post infection. To examine the role of IL-22 and IL-22BP in intestinal inflammation, we infected IL-22−/− and IL-22bp−/− mice by T.gondii. Despite a similar parasite load after infection (Fig. 1c), IL-22-deficient mice showed a reduced survival (Fig. 1b) and augmented inflammation characterized by increased IFNγ, IL-1β, TIMP1, and KC (Fig. 1f) as compared to the wild-type controls. Histological analysis revealed an increased intestinal epithelium damages in IL-22−/− mice as assessed by a severity score (Fig. 1d, e). Il18 expression was significantly reduced by T.gondii infection in B6 and was further reduced in IL-22−/− mice. Reg3β and Reg3γ expression, known to be regulated by IL-22, were diminished in infected IL-22−/− mice (Fig. 1g), as well as the expression of Anterior gradient homolog 2 (Agr2) (Figure 2C) that is produced by Goblet cells and is essential for mucin production.44 Expression of the Th17 pro-inflammatory cytokines Il17f and Il23a was increased in IL-22−/− mice after infection as compared to infected B6 controls (Fig. 1g).
IL-22−/− mice but not IL-22bp−/− are highly susceptible to T.gondii-induced inflammation. a B6 mice were infected by gavage with 35 cysts of T.gondii (76 K strain) and IL-22 and IL-22 bp transcripts were measured by Q-PCR in the intestinal mucosa at indicated time point post infection (n = 4–5 mice for each time point). b–g B6, IL-22−/− and IL-22bp−/− mice were infected (I) or not (NI) by 35 cysts of T.gondii. b Survival was recorded (n = 10–12 mice per group). c–g Intestinal samples collected 7 days post infection from B6, IL-22−/− and IL-22bp−/− infected (I) mice or non-infected mice (NI) (n = 4–6 mice per group). c Relative parasite load was determined by Q-PCR. d Representative photographs of HE staining performed on 3 µm-thick paraffin embedded sections of intestinal tissue. Scale bar=200 µm. e Cell infiltration, exudate, edema, and epithelium destruction was evaluated to determine the histological score, f IFNγ, IL-1β, TIMP1, and KC levels were determined by ELISA, and g Reg3b, Reg3g, Il23a and Il17f transcripts by Q-PCR. Values are representative of two independent experiments expressed as mean ± SEM. *, **, and *** refer to P < 0.05, P < 0.01 and P < 0.001, respectively.
IL-22 bioactivity may be regulated by the high-affinity soluble receptor, IL-22BP, and we hypothesized that in the absence of IL-22BP, might provide partial protection.21,22,23 However, IL-22bp−/− mice showed similar survival (Fig. 1b), histological features (Fig. 1d, e), inflammation (Fig. 1f), expression of antimicrobial Reg3β/γ and Th17 cytokines, Il23a, and Il17f (Fig. 1g) as the control mice. In the absence of Toxoplasma infection, Reg3β/γ expression was higher in IL-22bp−/− mice than in B6 mice suggesting a downregulation of IL-22-controlled Reg3β/γ by IL-22BP under steady-state conditions (Fig. 1g). Further, these data demonstrate a protective role of endogenous IL-22 in T. gondii-induced small intestine inflammation independent of parasite burden and that is not controlled by IL-22BP since the absence of IL-22BP has no effect on T. gondii-induced intestinal inflammation.
Microbiota exacerbates T. gondii-induced small intestinal inflammation
Since T.gondii causes epithelial cell damage at the intestinal entry site, it is hypothesized that translocated enteral microbes contribute to inflammation, which should be attenuated in germ-free mice. Indeed, Gram-negative bacteria play a role in Toxoplasma-induced small intestinal inflammation.36,37,38,39,40,41 To investigate whether Toxoplasma tachyzoites cause inflammation in the absence of intestinal bacteria, we performed T.gondii infection in germ-free mice.
In comparison to mice raised under specific pathogen free (SPF) conditions, T.gondii-induced mucosal inflammation was reduced with low levels of IFNγ, IL-1β, IL-22, TIMP1, KC, and MPO the intestine of infected germ-free (GF) B6 mice (Fig. 2a). Reg3β and Reg3γ expression was also reduced in the absence of microbiota (Fig. 2b), in accordance with previous studies.31,45,46 Despite using a unique inoculum for infection, a reduced parasite load was observed in GF mice compared to SPF mice (Fig. 2c). Histological analysis showed reduced, but still detectable epithelial damage and inflammation in the lamina propria of infected GF-B6 mice, suggesting direct damage of the intestinal epithelium by tachyzoites (Fig. 2d, e).
Small intestine inflammation occurs in the absence of bacteria, but is exacerbated by microbiota. a–d B6 mice under Specific Pathogen Free (SPF) or Germ-free (GF) conditions were infected (I) or not (NI) by 35 cysts of T.gondii and intestinal samples were collected 7 days post infection (n = 4–5 mice per group). a IFNγ, IL-1β, IL-22, TIMP1, KC, and MPO levels were determined by ELISA and b Reg3b and Reg3g transcripts by Q-PCR. c Relative parasite load was determined by Q-PCR. d Histological score of cell infiltration, exudate, edema and epithelium destruction was performed on 3 µm-thick paraffin embedded section after HE staining. e Representative HE staining of intestinal tissue. Scale bar=200 µm. Values are expressed as mean ± SEM. *, ** and *** refer to P < 0.05, P < 0.01 and P < 0.001, respectively.
However, even in the absence of microbiota some inflammatory markers such as IFNγ, IL-1β, MPO (Fig. 2a), and histological score (Fig. 2d) were significantly increased in infected GF-B6 mice in comparison to non-infected GF mice. Thus, the parasite T. gondii is able to cause injury of the intestinal epithelial barrier with inflammation in a germ-free environment, which is however exacerbated in the presence of normal intestinal microbiota.
The role of IL-22 in T.gondii-induced lesion in “sterile” intestinal inflammation was then investigated in GF-IL-22-/- in comparison to GF-B6 mice. While similar production of IFNγ, IL-1β, TIMP1, and LCN2, and histological features were detected in the gut of IL-22−/− and B6 mice under GF conditions after T. gondii infection, Reg3b and Reg3g expression were clearly reduced. This is similar to the reduced Reg3β/γ expression in T. gondii-infected IL-22-/- SPF mice (Fig. 1f). Thus, the similar levels of inflammation in IL-22−/− mice in the absence of microbiota suggest a contribution of IL-22-/--derived microbiota in T.gondii-induced small intestinal inflammation.
T. gondii-induced small intestinal inflammation is independent of Reg3β
Toxoplasma infection causes significant reduction in Paneth cells number47 and we confirmed that the absence of microbiota could prevent the Paneth cell loss as already observed by Raetz et al.41 Moreover, Reg3β/γ, a c-type lectin with antimicrobial activity, can be controlled by IL-22,12 as confirmed in Fig. 1g. The absence of IL-22, by down-regulating the production of Reg3β/γ, could thus influence the intestinal microbial composition which could lead to a dysbiotic state favoring T.gondii-induced small intestinal inflammation. We hypothesized that IL-22−/− susceptibility to T.gondii is linked to Reg3β downregulation. We next assessed whether Reg3β is necessary for the development of intestinal inflammation caused by T.gondii by using Reg3b−/− mice. IFNγ, IL-1β, IL-22, TIMP1, MPO and LCN2 intestinal levels were not significantly different in Reg3b−/− than in wild-type B6 mice 7 days post T.gondii infection (Fig. 3a). Moreover, similar destruction of intestinal epithelium and cell recruitment in the lamina propria were observed by histological analysis in Reg3b-/- and B6 mice (Fig. 3b, c). These results show that the absence of Reg3β is not sufficient to exacerbate the intestinal inflammation induced by T. gondii. Thus, the exacerbation of T. gondii-induced small intestinal inflammation seen in IL-22−/− mice is not reproduced by the sole absence of Reg3β.
Small intestinal inflammation is independent of Reg3b expression. a–d B6 and Reg3b−/− mice were infected (I) or not (NI) by 35 cysts of T.gondii and intestinal samples were collected 7 days post infection (n = 4–5 mice per group). a IFNγ, IL-1β, IL-22, TIMP1, MPO, and LCN2 levels were determined by ELISA b Histological score of cell infiltration, exudate, edema and epithelium destruction was performed on 3 µm-thick paraffin embedded section after HE staining. c Representative HE staining of intestinal tissue. Scale bar: 200 µm. Values are expressed as mean ± SEM. *, **, and *** refer to P < 0.05, P < 0.01 and P < 0.001, respectively.
Fecal transplantation of IL-22−/− microbiota to B6 mice does not transfer susceptibility to T.gondii
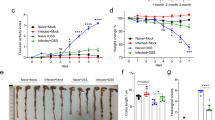
We further asked whether IL-22−/−-derived microbiota could contribute to small intestinal inflammation exacerbation. Indeed, T.gondii is able to induce Gram-negative bacteria overgrowth in the small intestine of WT mice.36,37,38,39,40,41 Thus, the pre-existing dysbiosis of IL-22−/− mice35 may also influence the pathogenesis of T.gondii-induced small intestinal inflammation. Several studies reported that a dysbiotic state predisposes to intestinal inflammation48,49,50 and we showed that in the absence of microbiota similar intestinal inflammation was detected in IL-22−/− and B6 mice. To examine whether IL-22-driven dysbiotic microbiota promotes Toxoplasma small intestinal inflammation in IL-22−/− mice, fecal transplantation from SPF-IL-22−/− mice (or SPF-B6 control mice) to GF-B6 mice was performed, followed by T.gondii oral infection after 3 weeks of fecal colonization. Interestingly, GF-B6 mice colonized by SPF B6-derived microbiota (B6>GF-B6) or SPF-IL-22−/− derived microbiota (IL-22−/−>GF-B6) presented similar production of IFNγ, KC, IL-22, TIMP1, MPO, LCN2 (Fig. 4a) and intestinal inflammation (Fig. 4b, c).
Fecal transplantation of IL-22−/−-derived microbiota is unable to transfer small intestinal inflammation to control B6 germ-free mice. a–d B6 mice under Germ-free (GF) conditions were colonized by B6 (B6>GF-B6) or IL-22−/− (IL-22−/−>GF-B6) fecal microbiota. 3 weeks post-colonization mice were infected (I) or not (NI) by 35 cysts of T.gondii and intestinal samples were collected 7 days post infection (n = 3–6 mice per group). a IFNγ, KC, IL-22, TIMP1, MPO and LCN2 levels were determined by ELISA and b Histological score of cell infiltration, exudate, edema and epithelium destruction was performed on 3 µm-thick paraffin embedded section after HE staining. c Representative HE staining of intestinal tissue. Scale bar=200 µm. d Microbial beta diversity, e Phylum (left panel) and family (panel right) composition were analysis in donor mice fecal samples and on feces collected 3 weeks after fecal transplantation by 16S rDNA sequencing. Values are expressed as mean ± SEM. *, **, and *** refer to P < 0.05, P < 0.01 and P < 0.001, respectively.
An analysis of microbiota diversity and composition 3 weeks after colonization confirmed a dysbiotic state in mice colonized by IL-22−/− microbiota similarly to the donor. Principal component analysis of Bray Curtis distance showed significant differences in the beta-diversity between GF-B6 mice colonized by IL-22−/− microbiota (IL-22−/−>GF-B6) or by B6 microbiota (B6>GF-B6) (p = 0.0001; Fig. 4d). Differences in microbiota composition observed in IL-22−/−>GF-B6 in comparison to B6>GF-B6 were also confirmed by LEfSe (Linear discriminant analysis effect size). Moreover, Proteobacteria mainly represented by Alcaligenaceae family were reduced and Firmicutes including Clostridiales, Ruminococcaceae and Turicibateraceae were increased in mice harboring IL-22−/− microbiota (Fig. 4e).
Thus, the transplantation of IL-22−/− mice derived microbiota to B6 mice did not transfer the phenotype of exacerbated intestinal inflammation seen in IL-22−/− mice suggesting that IL-22-deficiency itself is responsible for the pathology.
Discussion
Here we demonstrate that the intestinal inflammation observed in B6 mice in response to oral T. gondii 76K strain is exacerbated in the absence of IL-22. This phenotype seems independent of the reduced Reg3β antimicrobial peptide expression and of microbiota as fecal transplantation was not able to increase germ-free B6 susceptibility.
IL-22 has dual functions, either protective by repairing epithelium and controlling antimicrobial peptide production, or deleterious, contributing to inflammatory pathology, depending on tissue localization and cytokinic context. Indeed, IL-22 is involved in inflammation in lungs51 and skin52 but has protective effects in hepatic steatosis53 and experimental colitis induced by DSS or Citrobacter rodentium.12
Here we underline again the dual role of IL-22 in response to parasitic infection. Previous studies showed that IL-22 plays a deleterious role in response to high dose (50–100 cysts) of type II ME-49 T.gondii strain15,16 within the ileal compartment. In the present study, we report that IL-22 protects against small intestinal inflammation induced by low dose (35 cysts) of type II 76K T.gondii strain. We were surprised by the different susceptibility to closely related T.gondii strains. T.gondii was mostly described as inducing inflammation of the ileum for both 76,000 and ME-49 strains42,54 but we observed that essentially the jejunum was inflamed by the 76K strain, similarly to what was reported with Pru type II strain by Gregg et al.55 ME-49, Pru, and 76K T.gondii strains all belong to type II strains and the tissue area analyzed can explain some of the phenotypic differences. However the different outcomes might be due to yet unknown differences in virulence of the T.gondii strains and the infective dose used.6 The 76K stain used here, orally at low dose, induces small intestinal inflammation mimicking what can be observed in IBD. Furthermore, CD pathology in humans is associated with increased IL-22 expression, as in our model.7,8,9
Raetz et al.41 performed histological analysis of intestinal pathology caused by ME49 T.gondii strain and did not observe any differences between non-infected and infected germ-free mice. Our microscopic investigation combined with cytokine and protein analysis in the intestinal mucosa revealed a weak but distinct intestinal inflammation with production of IFNγ, IL-1β, MPO in response to T.gondii 76K strain in germ-free B6 mice.
The microbiota is required for intestinal injury and inflammation induced by 76K stain, but T.gondii per se can directly cause small intestinal damage and a weak intestinal inflammation under germ-free conditions. In accordance with our data, ME49 strain-induced intestinal inflammation is greatly increased in the presence of microbiota41,42,60 suggesting that intestinal inflammation is induced by both 76K and ME49 strains and exacerbated by microbiota. Interestingly, we observed a reduced parasite load in the absence of microbiota. This suggests that bacteria could maintain a close association between the parasite and the epithelial cells that improves parasite ability to invade the mucosa, regardless of the strain. On the contrary, in absence of microbiota, parasite could be eliminated easier.
We first hypothesized that IL-22-mediated mechanism, that protects mice from intestinal inflammation, was microbial composition control via the production of antimicrobial peptides as Reg3β. We observed that the inflammation was Reg3β-independent and that, despite the pre-existing dysbiosis induced by IL-22-deficiency,35 the microbiota did not contribute to IL-22−/− mice enhanced susceptibility to T.gondii-induced small intestinal inflammation. Indeed, the fecal transplantation from IL-22−/− to B6 mice did not transfer the higher susceptibility to the parasite.
As the inflammation process seems to be independent of dysbiosis, this suggests that other mechanisms are involved. Three hypotheses explaining how IL-22 controls intestinal pathology may be formulated:
-
(i)
IL-22 induces IL-18 production independently of the microbiota58 and we observed reduced IL-18 expression in IL-22−/− mice and reduced IL-18 expression during T.gondii infection suggesting that IL-22 confers protection though IL-18 regulation.
-
(ii)
Anterior gradient homolog 2 (AGR2) is a protein produced by goblet cells essential for mucin production44 and IL-22 has an important positive effect on goblet cell function.59 AGR2 expression was reduced after T.gondii infection in IL-22−/− mice in comparison to B6 infected mice suggesting that mucus production is affected in the absence of IL-22. In the intestine, the mucus layer is a barrier that can prevent the parasite from infecting epithelial cells as suggested in the literature.55 Thus, IL-22 could reduce inflammation by preventing intestinal cell invasion by parasites by regulating mucus production.
-
(iii)
Increased IL-17 might enhance T.gondii-induced small intestinal inflammation, as IL-17Ra-deficiency conferred protection to ileitis, as reported by Guiton et al.54 Our results showed an increased expression of Il23a and Il17f in IL-22 mice as compared to B6. It appears that compensatory mechanisms take place in the absence of IL-22, leading to increased IL-17 expression with exacerbation of inflammation. In addition, IL-17 can be affected by the microbiota composition. Indeed, deficiency in IL-22−/− allows the expansion of Segmented Filamentous Bacteria (SFB)56 (and our unpublished data), known to promote Th17 cells and IL-17 expression.57 Moreover, microbiota is necessary to the immune system development, including Th17 cell development.61 Thus, the absence of differences in the inflammatory state between B6 and IL-22-/- mice under germ-free conditions could be attributed, at least partly, to the impaired Th17 response. As IL17/IL-22 balance seems to play an essential role in the inflammation induced by the T.gondii 76 K strain, the IL-22-mediated mechanism that controls inflammation may be the increased IL-17 production via the control of microbiota composition.
In summary, we demonstrated that enteral T.gondii infection (76 K strain) causes intestinal epithelial injury and inflammation in the absence of microbiota. IL-22 plays a key role in regulating intestinal inflammation as IL-22-deficiency increases susceptibility to T.gondii infection.
Materials and methods
Mice
C57BL/6 J (B6) wild-type, IL-22−/−,62 IL-22bp−/−24 and Reg3b−/−63 (kindly provided by Iovanna JL) were bred in our specific pathogen free (SPF) animal facility at TAAM-CNRS, Orleans, France. B6 and IL-22 were derived by aseptic cesarean and maintained germ-free in sterile isolators at TAAM-CNRS. All KO mice were on the C57BL/6 J genetic background. Mice were maintained in a temperature-controlled (23 °C) facility with a strict 12 h light/dark cycle and were given free access to food and water. The experiments were performed with male mice aged 8–10 weeks with at least 3 weeks of adaptation time. All animal experimental protocols complied with the French ethical and animal experiments regulations (see Charte Nationale, Code Rural R 214–122, 214–124, and European Union Directive 86/609/EEC) and were approved by the “Ethics Committee for Animal Experimentation of CNRS Campus Orleans” (CCO), registered (No. 3) by the French National Committee of Ethical Reflexion for Animal Experimentation (CLE CCO 2013-1006).
Toxoplasma gondii infection
T.gondii 76K stain cysts were prepared by homogenization of brain tissue extracted from infected CBA/J mice that had been orally infected with 10 cysts eight weeks earlier. Numeration of cysts was performed by counting eight times 10 µL samples of this homogenate. The brain suspension containing cysts was diluted in order to contain 35 cysts for C57BL/6 J mice strain and 10 cysts for CBA/J mice strain per 200 µL and was administered intragastrically to each animal by gavage. C57BL/6J mice were orally infected with 35 cysts of the 76 K strain, as described above and necropsy was performed at day 1–8 post infection.
Fecal transplantation
Germ-free C57BL/6 J (B6) mice were generated at TAAM-CNRS by aseptic cesarean and keep under germ-free condition in sterile isolators. Transplantation by 200 µL of fecal homogenates from SPF-IL-22−/− mice or SPF B6 mice was administered intragastrically to each animal by gavage. After 3 weeks of colonization, mice were infected by 35 cysts of T.gondii (as mentioned above) in isolators. After 7 days of post infection mice were transferred aseptically to be killed and necropsied under sterile conditions.
ELISA
The small intestine (~100 mg) was homogenized in 1 mL of PBS and supernatants were tested for MPO, LCN2, IFNγ, IL-1β, TIMP1, IL-22, and KC/CXCL1 using commercial ELISA kits (R&D systems) according to the manufacturer’s instructions. Concentrations were normalized with organ weight and expressed in quantity per g of tissue.
RNA extraction and qPCR in the small intestine
Small intestine from control and infected B6 mice was collected, snap-freezed in liquid nitrogen and keeped at −80 °C. Total RNA was isolated from 100 mg of intestinal tissue homogenized with 1 mL of TRI Reagent® (Sigma) using TRIzol/Chloroform extraction. RNA was then precipitated in isopropanol, washed with 75% ethanol and resuspended in RNase-free water. Reverse transcription was performed on 1 µg of RNA using GoScript Reverse transcription system (Promega). Quantitative real-time PCR were realised on cDNA obtained using primers for IL-22, IL-22bp, Il17f, Il23a, Reg3b, Reg3g, Il18, and Agr2 (Qiagen), GoTaq® qPCR-Master Mix (Promega) and detected on a Stratagene Mx3005P (Agilent technologies). At the end of the PCR amplification, a DNA melting curve analysis was carried out to confirm the presence of a single amplicon. Gapdh expression was used for normalization of transcript levels. Relative mRNA levels were determined using (2-ΔΔCt) method, determined by comparing (i) the PCR cycle thresholds (Ct) for the gene of interest and Gapdh (ΔCt) and (ii) ΔCt values for treated and control groups (ΔΔCt).
Parasite load in intestine
Total DNA was extracted from intestinal tissue with NucleoSpin® Tissue kit (Macherey Nagel) according to manufacturer instructions. Parasites in tissue were quantified by a Q-PCR targeting a repetitive 529-bp cDNA fragment of T. gondii. Q-PCR were realized using GoTaq® qPCR-Master Mix (Promega) with 5 ng of DNA and 300 nM of each forward and reverse primer (Forward primer, 5′-AGGGACAGAAGTCGAAGGGG-3′; Reverse primer, 5′-GCAGCCAAGCCGGAAACATC-3′). A standard curve was performed using 156 pg-80 ng of tissue DNA containing T. gondii 76 K parasites to determin the parasite DNA amount.
Fecal DNA extraction and microbiota analysis
Fecal, genomic DNA was extracted from the weighted stool samples using a method that was previously described,64 which is based on the Godon DNA extraction method. 16 s rRNA gene sequencing of the fecal DNA samples was performed as previously described.64 Briefly, the V3-V4 region was amplified, and sequencing was performed on an Illumina MiSeq platform (GenoScreen, Lille, France). Raw paired-end reads were subjected to the following process: (1) quality-filtering using the PRINSEQ-lite PERL script by truncating the bases from the 3′ end that did not exhibit a quality below 30, on the basis of the Phred algorithm; (2) paired-end read assembly using FLASH (fast length adjustment of short reads to improve genome assemblies) (Schmieder, R. & Edwards, R). Quality control and preprocessing of metagenomic datasets65 with a minimum overlap of 30 bases and a 97% overlap identity; and (3) search and removal of both forward and reverse primer sequences using CutAdapt, with no mismatches allowed in the primer sequences. Assembled sequences for which perfect forward and reverse primers were not found were eliminated. Sequencing data were analyzed using the quantitative insights into microbial ecology (QIIME 1.9.1) software package. The sequences were assigned to OTUs using the UCLUST algorithm66 with a 97% threshold of pairwise identity and classified taxonomically using the Greengenes reference database.67 Rarefaction was performed (15,000 sequences per sample) and used to compare the abundance of the OTUs across samples. The alpha diversity was estimated using both the richness and evenness indexes (Chao1, Shannon or number of observed species). The beta diversity was measured by using the Bray Curtis distance matrix and was used to build the PCoA. The linear discriminant analysis (LDA) effect size (LEfSe) algorithm was used to identify taxa that were specific to the diet or treatment68
Histology
The small intestine was, fixed in 4% buffered formaldehyde and paraffin embedded under standard conditions. Tissue sections (3 µm) were stained with hematoxylin and eosin. The inflammatory cell infiltrate, exudate, edema and mucosal and epithelium destruction were assessed by a semi-quantitative score from 0 to 3 (with increasing extent) by two independent observers as described in Table 1. Paneth cell number was evaluated as described in Table 2.
Statistical analysis
Data were analyzed using Prism version 5 (Graphpad Software, San Diego, CA). The non-parametric Kruskal–Wallis test with Dunn’s multiple comparison test or the parametric one-way ANOVA test with multiple Bonferroni’s comparison test were used. Values are expressed as mean ± SEM. Statistical significance was defined at a p-value<0.05.
Change history
30 November 2018
The original version of this Article omitted the author Dr Mathias Chamaillard from the l’Institut de Pasteur, Lille, France. This has been corrected in both the PDF and HTML versions of the Article.
References
Kaser, A., Zeissig, S. & Blumberg, R. S. Inflammatory bowel disease. Annu. Rev. Immunol. 28, 573–621 (2010).
Egan, C. E., Cohen, S. B. & Denkers, E. Y. Insights into inflammatory bowel disease using Toxoplasma gondii as an infectious trigger. Immunol. Cell. Biol. 90, 668–675 (2012).
Liesenfeld, O. Oral infection of C57BL/6 mice 4 with Toxoplasma gondii: a new model of inflammatory bowel disease? J. Infect. Dis. 185(Suppl 1), S96–S101 (2002).
Cohen, S. B. & Denkers, E. Y. The gut mucosal immune response to Toxoplasma gondii. Parasite Immunol. 37, 108–117 (2015).
Buzoni-Gatel, D., Schulthess, J., Menard, L. C. & Kasper, L. H. Mucosal defences against orally acquired protozoan parasites, emphasis on Toxoplasma gondii infections. Cell Microbiol. 8, 535–544 (2006).
Munoz, M., Liesenfeld, O. & Heimesaat, M. M. Immunology of Toxoplasma gondii. Immunol. Rev. 240, 269–285 (2011).
Andoh, A. et al. Interleukin-22, a member of the IL-10 subfamily, induces inflammatory responses in colonic subepithelial myofibroblasts. Gastroenterology 129, 969–984 (2005).
Wolk, K. et al. IL-22 induces lipopolysaccharide-binding protein in hepatocytes: a potential systemic role of IL-22 in Crohn’s disease. J. Immunol. 178, 5973–5981 (2007).
Schmechel, S. et al. Linking genetic susceptibility to Crohn’s disease with Th17 cell function: IL-22 serum levels are increased in Crohn’s disease and correlate with disease activity and IL23R genotype status. Inflamm. Bowel. Dis. 14, 204–212 (2008).
Zenewicz, L. A. et al. Innate and adaptive interleukin-22 protects mice from inflammatory bowel disease. Immunity 29, 947–957 (2008).
Sugimoto, K. et al. IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J. Clin. Invest. 118, 534–544 (2008).
Zheng, Y. et al. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat. Med. 14, 282–289 (2008).
Ota, N. et al. IL-22 bridges the lymphotoxin pathway with the maintenance of colonic lymphoid structures during infection with Citrobacter rodentium. Nat. Immunol. 12, 941–948 (2011).
Hainzl, E. et al. Intestinal epithelial cell tyrosine kinase 2 transduces IL-22 signals to protect from acute colitis. J. Immunol. 195, 5011–5024 (2015).
Muñoz, M. et al. Interleukin (IL)-23 mediates Toxoplasma gondii-induced immunopathology in the gut via matrixmetalloproteinase-2 and IL-22 but independent of IL-17. J. Exp. Med. 206, 3047–3059 (2009).
Wilson, M. S. et al. Redundant and pathogenic roles for IL-22 in mycobacterial, protozoan, and helminth infections. J. Immunol. 184, 4378–4390 (2010).
Xie, M. H. et al. Interleukin (IL)-22, a novel human cytokine that signals through the interferon receptor-related proteins CRF2-4 and IL-22R. J. Biol. Chem. 275, 31335–31339 (2000).
Dumoutier, L., Van Roost, E., Colau, D. & Renauld, J. C. Human interleukin-10-related T cell-derived inducible factor: molecular cloning and functional characterization as an hepatocyte-stimulating factor. Proc. Natl Acad. Sci. USA 97, 10144–10149 (2000).
Lejeune, D. et al. Interleukin-22 (IL-22) activates the JAK/STAT, ERK, JNK, and p38 MAP kinase pathways in a rat hepatoma cell line. Pathways that are shared with and distinct from IL-10. J. Biol. Chem. 277, 33676–33682 (2002).
Jones, B. C., Logsdon, N. J. & Walter, M. R. Structure of IL-22 bound to its high-affinity IL-22R1 chain. Structure 16, 1333–1344 (2008).
Dumoutier, L., Lejeune, D., Colau, D. & Renauld, J. C. Cloning and characterization of IL-22 binding protein, a natural antagonist of IL-10-related T cell-derived inducible factor/IL-22. J. Immunol. 166, 7090–7095 (2001).
Xu, W. et al. A soluble class II cytokine receptor, IL-22RA2, is a naturally occurring IL-22 antagonist. Proc. Natl Acad. Sci. USA 98, 9511–9516 (2001).
Kotenko, S. V. et al. Identification, cloning, and characterization of a novel soluble receptor that binds IL-22 and neutralizes its activity. J. Immunol. 166, 7096–7103 (2001).
Huber, S. et al. IL-22BP is regulated by the inflammasome and modulates tumorigenesis in the intestine. Nature 491, 259–263 (2012).
Martin J. C. et al. IL-22BP is produced by eosinophils in human gut and blocks IL-22 protective actions during colitis. Mucosal Immunol. 2015.
Sugimoto, K. et al. IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J. Clin. Invest. 118, 534–544 (2008).
Wolk, K. et al. IL-22 increases the innate immunity of tissues. Immunity 21, 241–254 (2004).
Liang, S. C. et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J. Exp. Med. 203, 2271–2279 (2006).
Wolk, K. et al. IL-22 regulates the expression of genes responsible for antimicrobial defense, cellular differentiation, and mobility in keratinocytes: a potential role in psoriasis. Eur. J. Immunol. 36, 1309–1323 (2006).
Stallhofer, J. et al. Lipocalin-2 is a disease activity marker in inflammatory bowel disease regulated by IL-17A, IL-22, and TNF-α and modulated by IL23R genotype status. Inflamm. Bowel. Dis. 21, 2327–2340 (2015).
Cash, H. L., Whitham, C. V., Behrendt, C. L. & Hooper, L. V. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science 313, 1126–1130 (2006).
Sonnenburg, J. L., Chen, C. T. & Gordon, J. I. Genomic and metabolic studies of the impact of probiotics on a model gut symbiont and host. PLoS Biol. 4, e413 (2006).
Moucadel, V. et al. Cdx1 promotes cellular growth of epithelial intestinal cells through induction of the secretory protein PAP I. Eur. J. Cell Biol. 80, 156–163 (2001).
Vaishnava, S. et al. The antibacterial lectin RegIIIgamma promotes the spatial segregation of microbiota and host in the intestine. Science 334, 255–258 (2011).
Zenewicz, L. A. et al. IL-22 deficiency alters colonic microbiota to be transmissible and colitogenic. J. Immunol. 190, 5306–5312 (2013).
Bereswill, S. et al. Anti-inflammatory effects of resveratrol, curcumin and simvastatin in acute small intestinal inflammation. PLoS ONE 5, e15099 (2010).
Bereswill, S. et al. The impact of Toll-like-receptor-9 on intestinal microbiota composition and extra-intestinal sequelae in experimental Toxoplasma gondii induced ileitis. Gut Pathog. 6, 19 (2014).
Craven, M. et al. Inflammation drives dysbiosis and bacterial invasion in murine models of ileal Crohn’s disease. PLoS ONE 7, e41594 (2012).
Heimesaat, M. M. et al. Nucleotide-oligomerization-domain-2 affects commensal gut microbiota composition and intracerebral immunopathology in acute Toxoplasma gondii induced murine ileitis. PLoS ONE 9, e105120 (2014).
Molloy, M. J. et al. Intraluminal containment of commensal outgrowth in the gut during infection-induced dysbiosis. Cell. Host. Microbe 14, 318–328 (2013).
Raetz, M. et al. Parasite-induced TH1 cells and intestinal dysbiosis cooperate in IFN-γ-dependent elimination of Paneth cells. Nat. Immunol. 14, 136–142 (2013).
Heimesaat, M. M. et al. Gram-negative bacteria aggravate murine small intestinal Th1-type immunopathology following oral infection with Toxoplasma gondii. J. Immunol. 177, 8785–8795 (2006).
Darfeuille-Michaud, A. et al. High prevalence of adherent-invasive Escherichia coli associated with ileal mucosa in Crohn’s disease. Gastroenterology 127, 412–421 (2004).
Park, S. W. et al. The protein disulfide isomerase AGR2 is essential for production of intestinal mucus. Proc. Natl Acad. Sci. USA 106, 6950–6955 (2009).
Brandl, K. et al. Vancomycin-resistant enterococci exploit antibiotic-induced innate immune deficits. Nature 455, 804–807 (2008).
Keilbaugh, S. A. et al. Activation of RegIIIbeta/gamma and interferon gamma expression in the intestinal tract of SCID mice: an innate response to bacterial colonisation of the gut. Gut 54, 623–629 (2005).
Villeret, B. et al. Blockade of IL-1R signaling diminishes Paneth cell depletion and Toxoplasma gondii induced ileitis in mice. Am. J. Clin. Exp. Immunol. 2, 107–116 (2013).
Garrett, W. S. et al. Communicable ulcerative colitis induced by T-bet deficiency in the innate immune system. Cell 131, 33–45 (2007).
Elinav, E. et al. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell 145, 745–757 (2011).
Couturier-Maillard, A. et al. NOD2-mediated dysbiosis predisposes mice to transmissible colitis and colorectal cancer. J. Clin. Invest. 123, 700–711 (2013).
Besnard, A. G. et al. Dual Role of IL-22 in allergic airway inflammation and its cross-talk with IL-17A. Am. J. Respir. Crit. Care Med. 183, 1153–1163 (2011).
Ma, H. L. et al. IL-22 is required for Th17 cell-mediated pathology in a mouse model of psoriasis-like skin inflammation. J. Clin. Invest. 118, 597–607 (2008).
Yang, L. et al. Amelioration of high fat diet induced liver lipogenesis and hepatic steatosis by interleukin-22. J. Hepatol. 53, 339–347 (2010).
Guiton, R. et al. Interleukin 17 receptor signaling is deleterious during Toxoplasma gondii infection in susceptible BL6 mice. J. Infect. Dis. 202, 427–435 (2010).
Gregg, B. et al. Replication and distribution of Toxoplasma gondii in the small intestine after oral infection with tissue cysts. Infect. Immun. 81, 1635–1643 (2013).
Qiu, J. et al. Group 3 innate lymphoid cells inhibit T-cell-mediated intestinal inflammation through aryl hydrocarbon receptor signaling and regulation of microflora. Immunity 39, 386–399 (2013).
Ivanov, I. I. et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 139, 485–498 (2009).
Muñoz, M. et al. Interleukin-22 induces interleukin-18 expression from epithelial cells during intestinal infection. Immunity 42, 321–331 (2015).
Birchenough, G. M., Johansson, M. E., Gustafsson, J. K., Bergström, J. H. & Hansson, G. C. New developments in goblet cell mucus secretion and function. Mucosal Immunol. 8, 712–719 (2015).
Benson, A., Pifer, R., Behrendt, C. L., Hooper, L. V. & Yarovinsky, F. Gut commensal bacteria direct a protective immune response against Toxoplasma gondii. Cell. Host. Microbe 6, 187–196 (2009).
Kamada, N., Seo, S. U., Chen, G. Y. & Núñez, G. Role of the gut microbiota in immunity and inflammatory disease. Nat. Rev. Immunol. 13, 321–335 (2013).
Kreymborg, K. et al. IL-22 is expressed by Th17 cells in an IL-23-dependent fashion, but not required for the development of autoimmune encephalomyelitis. J. Immunol. 179, 8098–8104 (2007).
Lieu, H. T. et al. Reg2 inactivation increases sensitivity to Fas hepatotoxicity and delays liver regeneration post-hepatectomy in mice. Hepatology 44, 1452–1464 (2006).
Lamas, B. et al. CARD9 impacts colitis by altering gut microbiota metabolism of tryptophan into aryl hydrocarbon receptor ligands. Nat. Med. 22, 598–605 (2016).
Schmieder, R. & Edwards, R. Quality control and preprocessing of metagenomic datasets. Bioinformatics 27, 863–864 (2011).
Edgar, R. C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26, 2460–2461 (2010).
McDonald, D. et al. An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. ISME J. 6, 610–618 (2012).
Segata, N. et al. Metagenomic biomarker discovery and explanation. Genome Biol. 12, R60 (2011).
Acknowledgements
We thank Claire Mackowiak, Karine Jambou, Jérémy Paumier, Estelle Douin, Pascal Mauny, Tamara Durand and Elodie Desale for technical assistance. Support by Centre National de la Recherche Scientifique, the University of Orléans, la Région Centre (2009-00038261) and European Regional Development Fund (FEDER Bio-Target no. 2016-00110366). ACM received as a post-doctoral fellowship from l’Institut National de la Santé et de la Recherche Médicale (INSERM).
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: A.C.M., H.S., B.R. Performed the experiments: A.C.M., L.B., J.P.M., P.R., P.C., J.L.B., N.F., C.M., S.J. Analyzed the data: A.C.M., C.M., H.S., B.R. Wrote the paper: A.C.M., V.Q., B.R., H.S.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Couturier-Maillard, A., Froux, N., Piotet-Morin, J. et al. Interleukin-22-deficiency and microbiota contribute to the exacerbation of Toxoplasma gondii-induced intestinal inflammation. Mucosal Immunol 11, 1181–1190 (2018). https://doi.org/10.1038/s41385-018-0005-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41385-018-0005-8
This article is cited by
-
A framework for testing the impact of co-infections on host gut microbiomes
Animal Microbiome (2022)
-
The good and the bad about separation anxiety: roles of IL-22 and IL-22BP in liver pathologies
Seminars in Immunopathology (2021)