Abstract
Genetic factors contribute to the susceptibility of psychotic disorders, but less is known how they affect psychotic disease-course development. Utilizing polygenic scores (PGSs) in combination with longitudinal healthcare data with decades of follow-up we investigated the contributing genetics to psychotic disease-course severity and diagnostic shifts in the SUPER-Finland study, encompassing 10 403 genotyped individuals with a psychotic disorder. To longitudinally track the study participants’ past disease-course severity, we created a psychiatric hospitalization burden metric using the full-coverage and nation-wide Finnish in-hospital registry (data from 1969 and onwards). Using a hierarchical model, ranking the psychotic diagnoses according to clinical severity, we show that high schizophrenia PGS (SZ-PGS) was associated with progression from lower ranked psychotic disorders to schizophrenia (OR = 1.32 [1.23–1.43], p = 1.26e-12). This development manifested already at psychotic illness onset as a higher psychiatric hospitalization burden, the proxy for disease-course severity. In schizophrenia (n = 5 479), both a high SZ-PGS and a low educational attainment PGS (EA-PGS) were associated with increased psychiatric hospitalization burden (p = 1.00e-04 and p = 4.53e-10). The SZ-PGS and the EA-PGS associated with distinct patterns of hospital usage. In individuals with high SZ-PGS, the increased hospitalization burden was composed of longer individual hospital stays, while low EA-PGS associated with shorter but more frequent hospital visits. The negative effect of a low EA-PGS was found to be partly mediated via substance use disorder, a major risk factor for hospitalizations. In conclusion, we show that high SZ-PGS and low EA-PGS both impacted psychotic disease-course development negatively but resulted in different disease-course trajectories.
Similar content being viewed by others
Introduction
Psychotic disorders display considerable sharing of clinical symptoms and underlying genetic factors [1,2,3]. Consequently, the diagnostic separation is not always clear-cut, and it is common that an individual receives several diagnoses for different psychotic disorders over a lifetime. Such diagnostic shifts are often thought to reflect disease progression and changes in disease severity [4,5,6,7]. To account for these diagnostic shifts and to determine the main-lifetime diagnosis, hierarchical diagnostic models that rank the psychotic disorders are commonly used [8, 9]. Family-based genetic risk scores have recently been associated with specific psychiatric disease trajectories [10], but little is still known about how genetic factors contribute to disease-course development in psychotic disorders.
Here we studied the genetic contribution to disease-course development in 10,403 genotyped individuals diagnosed with a psychotic disorder (ICD10 equivalents: F20-29, F31, F32.3, F33.3) and up to 50 years of retrospective follow-up from high quality, full coverage, nation-wide Finnish healthcare registries [11]. Utilizing data from the Finnish Hospital Discharge Registry, which started in 1969, we calculated a psychiatric hospitalization burden metric to track disease severity throughout the individual disease-courses of the study participants’. We combined the disease-course severity metric with polygenic scores (PGSs) and leveraged the directionality of the hierarchical diagnostic model used in the SUPER-Finland protocol [12, 13] to investigate the genetic contribution to psychotic diagnosis progression and disease-course severity. This innovative way to utilize the longitudinal Finnish in-hospital register, with 50 years of follow-up, and in combination with genetic data provides a novel way for partitioning the genetic contribution for relevant risk factors and disease course outcome.
Methods
Study population
The SUPER-Finland study [13] is part of the Stanley Global Neuropsychiatric Genetics Initiative and includes 10 403 individuals, with active consent, and at least one episode of psychotic illness (ICD10 equivalents: F20-F29, F31, F32.3 and F33.3). The study recruitment (2016–2018) was nation-wide with the aim of collecting a representative sample of individuals with psychotic disorder in Finland. The recruitment and assessment took in the majority of cases place in the individuals’ treating unit (psychiatric hospitals, psychiatric out-patient clinics, and primary care units). Extensive questionnaire and interview data was collected at study inclusion, which was performed by psychiatrists and nurses specifically hired for the SUPER project. The questionnaire focused on self-reported well-being (present), quality of life, sleep, substance use, smoking and current self-reported diagnoses, while the interview focused on sociodemographic information and earlier stages in life (e.g school performance and disruptive behavior in youth). Also, supervised cognitive assessments was performed using the Cambridge Neuropsychological Test Automated Battery (CANTAB). All information from study inclusion was obtained by medical professionals but the protocol did not include a structured diagnostic interview due to time limitations. Full details of the study protocol have recently been published [13]. The study was approved by the Ethics Committee of the Hospital District of Helsinki and Uusimaa (Reference number 202/13/03/00/15). All participants were 18 years or older at inclusion and gave written informed consent. Details in Supplementary methods and https://thl.fi/en/web/thl-biobank/for-researchers/sample-collections/super-study.
Health care registry information
Information on specific diagnoses (ICD codes) and their time points were retrieved from the nation-wide Finnish National Care Register for Health Care and Register of Primary Health Care Visits (thl.fi/en/web/thlfi-en/statistics-and-data/data-and-services/register-descriptions). The clinical registry data includes information from (1) the hospital discharge registry (1969-); (2) specialized out-patient clinics (1998-) and (3) primary health care units (2011-) [Supplementary methods]. Because of the high quality of the Finnish health care registries [11], we only considered diagnoses recorded in the registries, and not self-reported diagnoses obtained at study inclusion. A main-lifetime psychotic diagnosis was set using a hierarchal diagnostic model contained within the SUPER-Finland study protocol [12, 13]. The model ranks the four major psychotic diagnosis based on clinical perception of severity as: (1) schizophrenia (SZ), n = 5 479; (2) schizoaffective disorder (SAD), n = 2124; (3) bipolar disorder (BD), n = 2 461; (4) major depressive disorder with psychotic features (psychotic MDD), n = 1542; (n refers to the number of individuals that ever received the diagnosis). In total, 8354 had a registry recorded diagnosis of at least one of these four major psychotic disorders, 1067 had unranked psychotic diagnoses and 405 had no registry-recorded psychotic diagnosis. These 405 individuals were treated for other psychiatric conditions and were included due to self-reported psychotic episodes, and/or due to misclassification at study inclusion [detailed in Supplementary methods, Table SM1 and Fig SM4]. Although the exact biological and clinical relationships between different psychotic disorders remain to be settled, similar hierarchical models have regularly been used, and on a group level SZ has been considered to be the top-ranking psychotic diagnosis [8, 9, 14]. SAD, although still a debated diagnosis, has often been viewed as an intermediate between BD and SZ [15,16,17], while psychotic MDD have been shown to be closest to BD [14].
Endpoints and definitions
Given that the presence of a psychotic illness was the main criterion for inclusion in the SUPER-Finland study, the age of psychotic illness onset was defined as the first registry recorded diagnosis indicating a psychotic illness [ICD code definitions in Supplementary methods]. Similarly, BD was defined to include all ICD codes for BD without specifying current psychotic symptoms (ICD10 equivalents: F30.2|F31.X). Substance use disorder (SUD, n = 1 763) was defined as having had any recorded diagnosis indicating a substance abuse or substance misuse apart from nicotine dependence (ICD10 equivalents: F10-F16 and F18-F19). Self-reported cannabis usage was cross-sectionally recorded at study inclusion but was not used for defining a SUD-endpoint due to absence of longitudinal information.
Psychiatric hospitalization burden
To track the study participants past disease-course severity a longitudinal metric was constructed based on the need for psychiatric hospital care using the Hospital Discharge Registry for which we had the longest follow-up (1969-01-01 to 2018-12-31). To account for changes in clinical practice over time and the steep decline in the number of psychiatric hospital beds during the last decades [18], the qualitative need for psychiatric in-patient care (hospital admissions of any length) for each individual and each year was recorded. The hospitalization data was then aligned for the first diagnosis of any psychotic disorder (set as the zero time point) to make the data comparable across individuals and take duration of illness into account. The hospitalization burden was calculated for each study participant as the fraction of years with at least one hospitalization primarily due to a psychiatric diagnosis for the first 15 years psychotic illness [detailed motivation for the construction of the metric in the Supplementary appendix]. To assess diagnostic progression, the hospitalization burden metric was used to assess the study participants’ disease severity for different timepoints during their disease-courses’ in relationship to their, at the time, current diagnoses.
Genotyping and imputation
The study participants were all genotyped using the Illumina Global Screening Array. Samples with poor genotype quality, ancestral outliers and samples that mismatched with the recorded sex were excluded [Supplementary methods]. Imputation was performed using a Finnish specific reference panel SISu version 3 (thl.fi/documents/3287543/3344176/THL+Biobank+Imputation+Panel.pdf) and only reliably imputed variants (INFO score >0.8) were included. After quality control the study included 9 826 individuals.
Construction of polygenic scores
Polygenic scores (PGSs), were constructed from the largest publicly available summary statistics at the time of publication for each trait of interest using MegaPRS [19] [Supplementary methods], which have shown good performance for psychiatric traits compared to other advanced PGS methods [20]. PGSs were constructed for seven psychiatric relevant traits: SZ, BD, major depressive disorder (MDD), intelligence, educational attainment (EA), CUD and alcohol dependence [2, 21,22,23,24,25,26].
Statistical models
Cox regression and linear/logistic regression were used in the analyses. In the genetic association models, sex, year of birth and the 10 first principal components were used as covariates. Transformations and analysis specific covariates were used when appropriate [Supplementary methods]. The significance level was adjusted for multiple testing and indicated in all analyses. All statistical analyses and figures were generated using R 4.1.2. A graphical overview of the study’s analysis approach can be found in Fig S1.
Results
Genetic contribution to psychotic disorder progression
To investigate the genetic contribution to the progression of psychotic disorders we leveraged a hierarchical model that ranked the four major psychotic disorders as: (1) SZ; (2) SAD; (3) BD and (4) psychotic MDD [“Methods”]. Of the study participants whose genotype data passed quality control, 8354 individuals had at least one of the four ranked major psychotic diagnoses recorded in the registries [“Methods”]. We defined diagnostic progression as a diagnostic shift from a lower ranked psychotic diagnosis to a higher ranked psychotic diagnosis. In total, 2605 individuals (31%) had received at least two of the four major psychotic diagnoses during their disease-course. The majority of these (n = 1561) had received the lowest ranked psychotic diagnosis first and most frequently had schizophrenia as their end-diagnosis (n = 926). To study diagnostic progression, we focused on these 926 individuals because they constituted the largest patient group with a uniform clinical endpoint (i.e progression from a lower ranked psychotic diagnosis to schizophrenia). We found that high SZ-PGS was associated with progression to schizophrenia from an initial lower ranked psychotic diagnosis (overall HR = 1.23 [1.15–1.31], p = 6.42e-10) [Fig. 1a]. The group that progressed to schizophrenia had on average a higher SZ-PGS than the lower ranked diagnostic groups (p = 0.0178 (SAD), p = 3.06e-16 (BD) and p = 1.95e-07 (psychotic MDD)) [Fig. 1b]. However, compared to the individuals who received schizophrenia as their first major psychotic diagnosis, the group that progressed to schizophrenia from a lower ranked diagnosis had a slightly lower SZ-PGS (mean difference: −0.10 SD, p = 0.0044). The lower ranked diagnostic groups (SAD, BD and psychotic MDD) characteristically present with more affective symptoms than in schizophrenia [27, 28], and the progression group did display a slightly higher MDD-PGS than other individuals with schizophrenia (mean difference: 0.11 SD, p = 0.0014).
a Progression from a lower ranked psychotic diagnosis to schizophrenia. The three panels show individuals who at some point in time had psychotic MDD, BD or SAD as their most severely ranked psychotic diagnosis but later progressed to schizophrenia compared to the individuals who remained at the corresponding lower ranked diagnoses. The results show that a larger proportion of individuals with a high SZ-PGS progressed to schizophrenia (combined HR = 1.23 [1.15-1.31], p = 6.42e-10). [SZ-PGS levels: Low = bottom <20%; Middle=20-60%; High = >80%; (1) Number of individuals who progressed to schizophrenia; (2) Number of individuals who remained at the specific lower ranked diagnosis.]. b Genetic map of SUPER participants. Polygenic composition of individuals with the four major psychotic diagnoses according to their hierarchical ranking, including the group that progressed to schizophrenia from an initial lower ranked diagnosis (orange). The psychotic and affective dimensions are proxied by the SZ-PGS and MDD-PGS respectively (mean PGS with error bars showing 95% Cl). The progression group displayed a higher SZ-PGS than any of the lower ranked diagnostic groups (p = 0.0178 (SAD), p = 3.06e-16 (BD) and p = 1.95e−07 (psychotic MDD)). [y-axis: SZ-PGS; x-axis: MDD-PGS; *=only individuals who had schizophrenia as their first major psychotic disorder].
Psychiatric hospitalization burden captures disease severity and diagnostic progression within psychotic disorders
Next, we investigated whether the disease-course severity of patients who progressed from a lower ranking psychotic disorder to schizophrenia differed from those who remained in their initial diagnostic group. For this purpose, we constructed a psychiatric hospitalization burden metric based on the yearly need for psychiatric hospital care as a method to track the participants past disease-course severity [“Methods”]. We observed that this metric conformed well with the hierarchical diagnostic severity ranking, where individuals with schizophrenia had the highest overall burden compared to the lower diagnostic groups [Fig. 2a]. Reassuringly, a high hospitalization burden showed consistently strong associations with outcomes often though to reflect general functioning [Fig. 2b], such as the need for supported housing and clozapine use, implying that the metric could serve as a proxy for disease-course severity.
a The psychiatric hospitalization burden for the four major psychotic disorders according to the diagnostic hierarchical model, where the highest ranked psychotic disorder was considered the current lifetime diagnosis. The y-axis shows the proportion hospitalized due to a psychiatric diagnosis in each group and the x-axis shows the age at event. Individuals with schizophrenia had the highest overall burden compared to the lower ranking diagnoses (β = 0.65, p = 3.26e-186). b Associations between endpoints often thought to reflect general functioning and their association to the average yearly psychiatric hospitalization burden. The results show that an increased hospitalization burden was associated with a poor outcome for all endpoints, suggesting that the measurement can serve as a proxy to track disease-severity. Note, the analysis was constrained to individuals with schizophrenia (n = 5479) to not be influence by the cohort’s diagnostic composition. (Parameters have been named to make the direction of effect intuitive; n denotes the number of individuals with non-missing data for the specific endpoint; *Adjusted Paired Associates Learning (PAL) errors, a part of the CANTAB suit to assess cognitive function.).
We then compared the psychiatric hospitalization burden of the individuals that progressed to schizophrenia (n = 926) with those who remained at the lower ranked diagnoses (psychotic MDD, n = 507; BD, n = 1 494; SAD, n = 874). To understand how the hospitalization burden evolved from the start of the disease-course, we aligned the hospitalization data according to the age at onset of any psychotic disorder [“Methods”]. The comparison revealed that the individuals who later progressed to schizophrenia had a substantially higher hospitalization burden than individuals with lower ranked diagnoses. Moreover, already from the first recorded sign of psychosis, their hospitalization burden was as high as individuals who had schizophrenia as their first major psychotic diagnosis [Fig S2], despite having a delayed schizophrenia diagnosis (median delay = 5.6 years later, p = 1.02e-79) [Table 1]. The age at illness onset for the individuals that progressed to schizophrenia did not differ from the individuals who directly received a schizophrenia diagnosis (median difference: 0.1 years) but were significantly lower compared to individuals with any of the other three major psychotic diagnoses [Table 1]. This suggested that the overall severity of illness in the group that progressed to schizophrenia was similar to other individuals with schizophrenia already from disease-course start. The delay in schizophrenia diagnosis for the progression group was seen across decades, supporting the analysis strategy, and suggesting that the results are not a reflection of changing clinical diagnostic practices over time [Fig SA7, supplemental appendix]. Finally, we observed that compared to males, females were more likely to progress from a lower ranked diagnosis to schizophrenia than to receive schizophrenia as their first major psychotic disorder (OR = 1.72 [1.49–1.99], p = 1.08e-13) [Table 1], suggesting a potential diagnostic sex bias.
Genetic contribution to psychiatric hospitalization burden in schizophrenia
We next investigated the genetic contribution to psychiatric hospitalization burden. Since the need for psychiatric hospital care were significantly different between the four specific psychotic diagnoses, we confined the analyses to individuals with schizophrenia (n = 5479) to avoid biases due to the study’s diagnostic composition. We evaluated the relationship between the yearly need of psychiatric hospitalization and PGSs for seven traits deemed relevant [“Methods”]. Among individuals with schizophrenia, the SZ-PGS was significantly associated with hospitalization burden over an individual’s adult life-course (β = 0.067, p = 1.58e-06). This was true also after aligning the data for the time-point of psychotic illness onset (β = 0.054, p = 1.00e-04) [Fig. 3a, b]. Because the underlying GWAS used to construct the SZ-PGS likely is enriched for treatment resistant cases, we also performed the analysis in non-clozapine users (n = 3377, β = 0.063, p = 4.81e-04) and observed that the association between the SZ-PGS and hospitalization burden remained virtually unchanged [2]. In addition to the SZ-PGS, the MDD-PGS also showed a strong influence on psychiatric hospitalization burden within schizophrenia individuals [Fig S3]. However, in contrast to high SZ-PGS, high MDD-PGS was associated with having been hospitalized for a non-psychotic depression and/or anxiety diagnoses (OR = 1.17 [1.11–1.24], p = 1.24e-07), which were common primary reasons for hospital admissions, both before and after the psychotic illness onset.
a Heatmap displaying the proportion hospitalized due to a psychiatric diagnosis each year (colored) in relationship to SZ-PGS deciles (y-axis) and age (x-axis). In individuals with schizophrenia a high SZ-PGS was associated with an increased need of psychiatric hospital care (β = 0.067, p = 1.58e-06). b The proportion hospitalized for a primary psychiatric diagnosis in relation to SZ-PGS. The hospitalization data was aligned for the time-point of first recorded psychotic illness. A high SZ-PGS was associated with an increased need of hospital care post psychotic illness onset (β = 0.054, p = 1.00e-04). [SZ-PGS strata: Low = <20% (n = 1 096); Middle = 20–60% (n = 3 287); High = >80% (n = 1 096); total n = 5 479].
Intriguingly, the EA-PGS had the strongest association to psychiatric hospitalization burden following the onset of any psychotic disorder (β = −0.084, p = 4.53e-10) [Fig S3]. We took special interest in the EA-PGS because it was independent from the SZ-PGS (r = −0.017) [Fig S4], which suggested that the EA-PGS reflected a different mechanism of action that contributed to the need of psychiatric hospital care.
The EA-PGS and the SZ-PGS displayed different hospital usage profiles
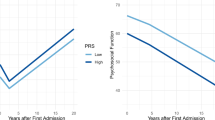
The apparent independent effects of SZ-PGS and EA-PGS on the psychiatric hospitalization burden let us to characterize the patterns of hospital admissions more closely. The analysis revealed that the SZ-PGS and the EA-PGS were associated with distinct profiles of hospital usage [Fig. 4]. While both a high SZ-PGS and a low EA-PGS were associated with a longer total time spent in hospital (β = 0.070, p = 2.47e-07, β = 0.049, p = 1.99e-04 respectively), the composition of the hospital stays was different. A high SZ-PGS associated with longer individual stays in the hospital (β = 0.051, p = 3.30e-04). A low EA-PGS, in contrast, associated with shorter (β = −0.054, p = 6.05e-05), but more frequent, hospital stays. Taken together, the hospital usage profile of individuals with a high SZ-PGS showed a pattern more likely to reflect a disorder with increased disease-severity and greater need of medical care. On the other hand, the hospital usage profile associated with a low EA-PGS resembled a pattern often called a “revolving door” phenomenon, characterized by short and frequent visits, regularly observed when drug and/or substance abuse is present [29, 30].
The upper panels (a, b) display the relationship between the EA-PGS and the SZ-PGS and the total length of stay for the first 15 years post psychotic illness onset. The two lower panels (c + d) show the relationship between the EA-PGS and the SZ-PGS and the median length of each separate hospital visit for the same time-period. The EA-PGS and the SZ-PGS associated with two distinctly different hospitalization profiles. A low EA-PGS associates with a longer total time spent at hospital, but the total time was composed of shorter, more frequent visits, while a high SZ-PRS associated with longer total time spent at hospital and longer individual hospital stays. [PGS strata: Low = <20% (n = 1096); Middle = 20–60% (n = 3287); High = >80% (n = 1096); total n = 5479].
Substance use disorder partly mediates the EA PGS’s effect on psychiatric hospitalization burden
We next wondered about the potential reasons for the observed differences in the patterns of hospital stays associated with SZ-PGS and EA-PGS. Because substance use disorder (SUD) is known to be related to a revolving door pattern, and is a common comorbidity in psychotic disorders [31], we examined whether the EA-PGS’s hospitalization pattern also was related to SUD in our study. For this purpose, we studied the association of SUD in individuals with schizophrenia (n = 1763, “Methods”) to the pattern of hospital treatment periods. In line with previous reports [32,33,34,35], a diagnosis of SUD had a major impact on the psychiatric hospitalization burden (β = 0.50, p = 2.95e-65). Interestingly, the association between the SUD-endpoint and the EA-PGS was far stronger (OR = 0.68 [0.64–0.72], p = 1.37e-35) than for SZ-PGS (OR = 0.99 [0.94–1.056) or the other assessed PGSs [Fig S5]. The EA-PGS also displayed a strong association with self-reported cannabis usage that was measured at study inclusion, while the SZ-PGS was not associated with either the SUD-endpoint (p = 0.85) or self-reported cannabis usage (p = 0.52) [Fig S6]. Importantly, we observed that the EA-PGS displayed the same effects in a non-psychiatric population (n = 30,544) in the Finnish nation-wide FinnGen study, where SUD were consistently associated to the EA-PGS but not with the SZ-PGS [Supplementary appendix and Supplementary methods]. Further, we observed that outcomes that were primarily associated with the EA-PGS tended to share the EA-PGS’s hospitalization usage profile, and vice versa for the SZ-PGS [Fig S7]. The revolving door pattern was most evident for individuals with SUD, who had a much shorter median length of stay (21 vs 41 days, p = 4.16e-64) while still having a longer total time spent in the hospital (β = 0.22, p = 6.65e-15). The SUD-endpoint showed a time-dependent effect on psychiatric hospitalizations, suggesting it might act as a direct effector on the need for psychiatric hospital care, and not just a marker for a poor outcome [Fig S8].
The intimate relationship between the SUD-endpoint and the EA-PGS lead us to hypothesize that the SUD-endpoint could act as a mediator for the EA-PGS’s effect on psychiatric hospitalizations. This hypothesis was further supported by a complete attenuation of the association between the EA-PGS and psychiatric hospitalization burden when all individuals with the SUD-endpoint were excluded from the analysis (β = −0.020, p = 0.23, after SUD exclusion) [Fig S9]. We used structural equation modeling (SEM) to assess potential causal directions between the EA-PGS, SUD-endpoint and psychiatric hospitalizations. The SEM analysis gave strong support that the EA PGS’s effect on psychiatric hospitalization burden was, indeed, partly mediated via its effect on the SUD-endpoint (β = −0.040, p = 1.03e-24) [Fig S10]. In the mediation model the highest acquired educational level for each individual was also included as a possible second mediator, given its apparent and immediate relationship with the EA-PGS. However, unlike the SUD-endpoint, the highest acquired educational level did not display a noticeable temporal effect on psychiatric hospitalization burden [Fig S11]. Excluding the acquired educational level from the model marginally strengthened the EA-PGS’s indirect mediation effect via the SUD-endpoint (β = −0.044, p = 1.66e-27).
Discussion
We investigated the genetic contribution to diagnostic progression and disease severity within the spectrum of psychotic diagnoses in the SUPER-Finland study, which builds on nation-wide, full coverage, high-quality medical healthcare registries in Finland. While PGSs for psychiatric disorders are still insufficient for screening purposes in the general population [2, 36] their use for prognostic prediction would meet a great clinical need for patients with a psychotic illness. Hitherto, only a few clinical predictors have been associated with a poor outcome following first psychotic episode [37]. Here, using this unique dataset, with decades of medical follow-up, we were able to characterize the disease-course development in an innovative way by longitudinally tracking past disease-course severity and investigating the polygenetic architecture contributing to disease outcome. High SZ-PGS was associated with progression from a lower ranked psychotic disorder to schizophrenia and a greater need for psychiatric hospital care in individuals with schizophrenia. The EA-PGS had the largest impact on psychiatric hospitalization burden. The effect was in part explained by its influence on the risk of acquiring SUD, and it was independent from the effect of SZ-PGS. So, although both a high SZ-PGS and a low EA-PGS increased the total psychiatric hospitalization burden over time, the effects were due to different reasons. This conclusion was also supported by evidence that the EA-PGS displayed the same effects in a non-psychiatric population. High SZ-PGS was associated with a hospital usage profile more likely to reflect severe psychotic disease, while low EA-PGS was associated with a hospitalization pattern regularly observed when SUD is present [29, 30]. These results are in support of a future potential utility for polygenic scores to help clinicians in prognostic guidance and to help in planning supportive efforts, especially focusing on SUD.
Diagnostic progression of psychotic disorders
The degree and quality of psychotic symptoms can progress over the disease-course and the diagnosis is therefore often adjusted over time [4]. Our findings suggest, however, that the individuals who later will progress to schizophrenia from an initial lower ranked psychotic disorder have a similar need for psychiatric hospital care as other individuals with schizophrenia already from the start of their psychotic illness. This was in stark contrast to the significantly lower need for hospital care observed for individuals who remained at a lower ranked diagnosis. The group that progressed to schizophrenia had their psychotic illness onset at the same age as other individuals with schizophrenia but received their schizophrenia diagnosis substantially later (diagnosis delay was almost 6 years). Although these results could be affected by changing diagnostic practices over time, the schizophrenia diagnosis delay for the progression group was seen across all studied time periods (Fig SA7). The individuals who progressed to schizophrenia also had higher SZ-PGS than individuals who remained at lower ranked psychotic diagnoses, together suggesting that initial diagnostic difficulties might partially explain these findings or at least that their disease-course development was largely determined already at the start of first psychotic illness onset. Interestingly a strong sex imbalance (p = 1.08e-13) was seen between the progression group (53.1% females/46.9% males), and the group that received schizophrenia as the first major psychotic disorder (40.8% females/59.2% males). This observation could potentially, in part, explain why many studies have found women to be older at the onset of schizophrenia compared to males [38, 39]. However, the progression group had a slightly higher MDD-PGS than other individuals with schizophrenia, signifying that their symptomatology could have differed and possibly involved more affective symptoms.
Psychiatric hospitalization burden in schizophrenia
Recent studies in hypertension and age-related macular degeneration have shown that common genetic variants can add prognostic value, beyond their ability to cross-sectionally predict the trait [40, 41]. In schizophrenia, we showed that individuals with high SZ-PGS had an increased psychiatric hospitalization burden, and that they displayed a hospitalization usage profile indicative of a more severe disease. The results suggest that the genetic factors that are important for the development of schizophrenia also influence the disease-course. However, the schizophrenia GWAS used to construct the PGS [2] were likely enriched for severe cases and included the CLOZUK cohort with more than 5000 treatment resistant cases (proxied by clozapine usage). Treatment resistance schizophrenia have recently been demonstrated to be a trait with independent heritability, and that a high clozapine dose associates with high SZ-PGS [42, 43]. However, the genetic architecture for treatment resistant schizophrenia cases has been shown to be very similar (rg=0.954) to non-treatment resistant cases, suggesting that a bias would only be moderate [44]. Further, treatment resistance is difficult to measure in registry-based research [45], and although a prescription of clozapine is often used as a proxy for treatment resistance, there is wide geographical variation in clozapine use suggesting that not all individuals who would benefit from clozapine treatment actually receive it [46]. In a sensitivity analysis we also showed that the association between the SZ-PGS and increased need for psychiatric hospitalizations remained in non-clozapine users.
The EA-PGS had the largest influence on psychiatric hospitalization burden
A high EA-PGS is known to associate with positive psychosocial outcomes, both within psychiatric and somatic disorders [23, 47], and in our study the EA-PGS had the largest influence on psychiatric hospitalization burden. The EA-PGS, unlike the SZ-PGS, was associated with frequent short visits in resemblance with the SUD-related revolving door phenomenon. Previously, low performance in primary school has been causally linked with future drug abuse [48]. In line with these observations, we observed that the SUD-endpoint, which had a major impact on the hospitalization burden, predominantly associated with the EA-PGS, but not with the SZ-PGS (p = 0.85). Also, after excluding all individuals with a SUD diagnosis (n = 1763), the EA-PGS were no longer associated with hospital burden (p = 0.23, Fig S9). The SEM analysis showed a strong indirect effect of the EA-PGS via the SUD-endpoint and together the results form credible support that the association between a low EA-PGS and increased psychiatric hospitalization burden was mediated in part by SUD. That a low EA-PGS was associated with an unfavorable outcome via SUD, independent of the SZ-PGS, indirectly suggest that initiatives focusing on treatment and/or prevention of SUD could prevent frequent hospitalizations and improve the outcome of people with dual diagnoses. We draw this conclusion because our results do not support SUD to be a consequence of a more severe psychotic disease, but instead that SUD leads to a poor hospitalization outcome independently of an individual’s genetic liability to schizophrenia.
Study limitations
Although this study has several strengths, foremost due to the use of nation-wide, full coverage, medial healthcare registry data in a sizable cohort, there are some important limitations. The study includes longitudinal health-care register data over a multi-generational cohort of individuals (born between 1927–2000) that have been subject to changing clinical practices over the last 50 years. In addition, there are inherent stratification biases due to the recruitment. First, because we require an occurrence for a psychotic disorder, or even a diagnosis of schizophrenia, we are at risk for index event bias. However, when focusing on hospitalization outcomes, we confided the analysis to schizophrenia cases to make the results interpretable. Further, all study participants needed to be alive at study inclusion (2016–2018) and capable to consenting, resulting in potential underrepresentation of older individuals, while all young study participants needed to already have received a psychotic diagnosis to be included. This may impact comparisons across generations because of different time-constrains in their individual disease course-development. Our hospitalization metric aimed to mitigate these generational differences, but this issue can never fully be overcome.
Summary
In summary, we show that individuals with high SZ-PGS were more likely to progress from another major psychotic diagnosis to schizophrenia and had an increased need for psychiatric hospital care. Individuals with low EA-PGS also had an increased need for psychiatric hospital care, but not for the same reasons as individuals with a high SZ-PGS. Instead, we find support that the association between a low EA-PGS and increased psychiatric hospitalizations was partly mediated by substance use disorder, resulting in a specific disease-trajectory that potentially could be targeted with preventive efforts.
Data availability
This study includes individual genotypes and sensitive health care data. We are not allowed to transfer the data outside the secure environments at the Institute for Molecular Medicine Finland, Helsinki, Finland and The Stanley Center, Broad Institute of MIT and Harvard, Cambrigde, USA. However, data access can be managed in collaboration with the authors. For data access requests, contact OP.
References
Barkhuizen W, Pain O, Dudbridge F, Ronald A. Genetic overlap between psychotic experiences in the community across age and with psychiatric disorders. Transl Psychiatry. 2020;10:86.
Trubetskoy V, Pardiñas AF, Qi T, Panagiotaropoulou G, Awasthi S, Bigdeli TB, et al. Mapping genomic loci implicates genes and synaptic biology in schizophrenia. Nature. 2022;604:502–8.
Bulik-Sullivan B, Finucane HK, Anttila V, Gusev A, Day FR, Loh PR, et al. An atlas of genetic correlations across human diseases and traits. Nat Genet. 2015;47:1236–41.
Bromet EJ, Kotov R, Fochtmann LJ, Carlson GA, Tanenberg-Karant M, Ruggero C, et al. Diagnostic shifts during the decade following first admission for psychosis. Am J Psychiatry. 2011;168:1186–94.
Hung YN, Yang SY, Kuo CJ, Lin SK. Diagnostic consistency and interchangeability of schizophrenic disorders and bipolar disorders: a 7-year follow-up study. Psychiatry Clin Neurosci. 2018;72:180–8.
Fusar-Poli P, Cappucciati M, Rutigliano G, Heslin M, Stahl D, Brittenden Z, et al. Diagnostic stability of ICD/DSM first episode psychosis diagnoses: meta-analysis. Schizophr Bull. 2016;42:1395–406.
Kendler KS, Ohlsson H, Sundquist J, Sundquist K. Family genetic risk scores and the genetic architecture of major affective and psychotic disorders in a Swedish National Sample. JAMA Psychiatry. 2021;78:735–43.
Cardno AG, Owen MJ. Genetic relationships between schizophrenia, bipolar disorder, and schizoaffective disorder. Schizophr Bull. 2014;40:504–15.
Zammit S, Allebeck P, David AS, Dalman C, Hemmingsson T, Lundberg I, et al. A longitudinal study of premorbid IQ score and risk of developing schizophrenia,bipolar disorder, severe depression, and other nonaffective psychoses. Arch Gen Psychiatry. 2004;61:354–60.
Kendler KS, Ohlsson H, Sundquist J, Sundquist K. Relationship of family genetic risk score with diagnostic trajectory in a Swedish National Sample of incident cases of major depression, bipolar disorder, other nonaffective psychosis, and schizophrenia. JAMA Psychiatry. 2023;80:241–9.
Sund R. Quality of the Finnish Hospital Discharge Register: a systematic review. Scand J Public Health. 2012;40:505–15.
Ahti J, Kieseppä T, Suvisaari J, Suokas K, Holm M, Wegelius A, et al. Differences in psychosocial functioning between psychotic disorders in the Finnish SUPER study. Schizophr Res. 2022;244:10–7.
Lähteenvuo M, Ahola-Olli A, Suokas K, Holm M, Misiewicz Z, Jukuri T, et al. Cohort profile: SUPER-Finland—the Finnish study for hereditary mechanisms of psychotic disorders. BMJ Open. 2023;13:e070710.
Nietola M, Heiskala A, Nordstrom T, Miettunen J, Korkeila J, Jaaskelainen E. Clinical characteristics and outcomes of psychotic depression in the Northern Finland Birth Cohort 1966. Eur Psychiatry. 2018;53:23–30.
Tamminga CA, Ivleva EI, Keshavan MS, Pearlson GD, Clementz BA, Witte B, et al. Clinical Phenotypes of Psychosis in the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP). Am J Psychiatry. 2013;170:1263–74.
Kendler KS, McGuire M, Gruenberg AM, Walsh D. Examining the validity of DSM-III-R schizoaffective disorder and its putative subtypes in the Roscommon Family Study. Am J Psychiatry. 1995;152:755–64.
Benabarre A, Vieta E, Colom F, Martínez-Arán A, Reinares M, Gastó C. Bipolar disorder, schizoaffective disorder and schizophrenia: epidemiologic, clinical and prognostic differences. Eur Psychiatry. 2001;16:167–72.
CEPHOS-LINK (EU project). (2023). https://thl.fi/en/web/thlfi-en/research-and-development/research-and-projects.
Zhang Q, Privé F, Vilhjálmsson B, Speed D. Improved genetic prediction of complex traits from individual-level data or summary statistics. Nat Commun. 2021;12:4192.
Ni G, Zeng J, Revez JA, Wang Y, Zheng Z, Ge T, et al. A Comparison of Ten Polygenic Score Methods for Psychiatric Disorders Applied Across Multiple Cohorts. Biol Psychiatry. 2021;90:611–20.
Mullins N, Forstner AJ, O’Connell KS, Coombes B, Coleman JRI, Qiao Z, et al. Genome-wide association study of more than 40,000 bipolar disorder cases provides new insights into the underlying biology. Nat Genet. 2021;53:817–29.
Wray NR, Ripke S, Mattheisen M, Trzaskowski M, Byrne EM, Abdellaoui A, et al. Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nat Genet. 2018;50:668–81.
Okbay A, Wu Y, Wang N, Jayashankar H, Bennett M, Nehzati SM, et al. Polygenic prediction of educational attainment within and between families from genome-wide association analyses in 3 million individuals. Nat Genet. 2022;54:437–49.
Savage JE, Jansen PR, Stringer S, Watanabe K, Bryois J, de Leeuw CA, et al. Genome-wide association meta-analysis in 269,867 individuals identifies new genetic and functional links to intelligence. Nat Genet. 2018;50:912–9.
Johnson EC, Demontis D, Thorgeirsson TE, Walters RK, Polimanti R, Hatoum AS, et al. A large-scale genome-wide association study meta-analysis of cannabis use disorder. Lancet Psychiatry. 2020;7:1032–45.
Walters RK, Polimanti R, Johnson EC, McClintick JN, Adams MJ, Adkins AE, et al. Transancestral GWAS of alcohol dependence reveals common genetic underpinnings with psychiatric disorders. Nat Neurosci. 2018;21:1656–69.
Ruderfer DM, Ripke S, McQuillin A, Boocock J, Stahl EA, Whitehead Pavlides JM et al. Genomic Dissection of Bipolar Disorder and Schizophrenia, Including 28 Subphenotypes. Cell. 2018;173:1705-15.e16.
Lindenmayer J-P, Kaur A. Antipsychotic management of schizoaffective disorder: a review. Drugs. 2016;76:589–604.
Haywood TW, Kravitz HM, Grossman LS, Cavanaugh JL Jr, Davis JM, Lewis DA. Predicting the “revolving door” phenomenon among patients with schizophrenic, schizoaffective, and affective disorders. Am J Psychiatry. 1995;152:856–61.
Di Giovanni P, Di Martino G, Zecca IAL, Porfilio I, Romano F, Staniscia T. The revolving door phenomenon: psychiatric hospitalization and risk of readmission among drug-addicted patients. Clin Ter. 2020;171:e421–e4.
Hunt GE, Large MM, Cleary M, Lai HMX, Saunders JB. Prevalence of comorbid substance use in schizophrenia spectrum disorders in community and clinical settings, 1990–2017: Systematic review and meta-analysis. Drug Alcohol Depend. 2018;191:234–58.
Gonçalves-Pinho M, Bragança M, Freitas A. Psychotic disorders hospitalizations associated with cannabis abuse or dependence: A nationwide big data analysis. Int J Methods Psychiatr Res. 2020;29:e1813.
Winklbaur B, Ebner N, Sachs G, Thau K, Fischer G. Substance abuse in patients with schizophrenia. Dialogues Clin Neurosci. 2006;8:37–43.
Opsal A, Kristensen Ø, Larsen TK, Syversen G, Rudshaug BEA, Gerdner A, et al. Factors associated with involuntary admissions among patients with substance use disorders and comorbidity: a cross-sectional study. BMC Health Serv Res. 2013;13:57.
Opsal A, Clausen T, Kristensen O, Elvik I, Joa I, Larsen TK. Involuntary hospitalization of first-episode psychosis with substance abuse during a 2-year follow-up. Acta Psychiatr Scand. 2011;124:198–204.
Lewis CM, Vassos E. Polygenic risk scores: from research tools to clinical instruments. Genome Med. 2020;12:44.
Ajnakina O, Stubbs B, Francis E, Gaughran F, David AS, Murray RM, et al. Hospitalisation and length of hospital stay following first-episode psychosis: systematic review and meta-analysis of longitudinal studies. Psychol Med. 2020;50:991–1001.
Leung A, Chue P. Sex differences in schizophrenia, a review of the literature. Acta Psychiatr Scand Suppl. 2000;401:3–38.
Takahashi S, Matsuura M, Tanabe E, Yara K, Nonaka K, Fukura Y, et al. Age at onset of schizophrenia: Gender differences and influence of temporal socioeconomic change. Psychiatry Clin Neurosci. 2000;54:153–6.
Kurniansyah N, Goodman MO, Kelly TN, Elfassy T, Wiggins KL, Bis JC, et al. A multi-ethnic polygenic risk score is associated with hypertension prevalence and progression throughout adulthood. Nat Commun. 2022;13:3549.
Schmitz-Valckenberg S, Fleckenstein M, Zouache MA, Pfau M, Pappas C, Hageman JL, et al. Progression of Age-Related Macular Degeneration Among Individuals Homozygous for Risk Alleles on Chromosome 1 (CFH-CFHR5) or Chromosome 10 (ARMS2/HTRA1) or Both. JAMA Ophthalmol. 2022;140:252–60.
Pardinas AF, Smart SE, Willcocks IR, Holmans PA, Dennison CA, Lynham AJ, et al. Interaction Testing and Polygenic Risk Scoring to Estimate the Association of Common Genetic Variants With Treatment Resistance in Schizophrenia. JAMA Psychiatry. 2022;79:260–9.
Kappel DB, Legge SE, Hubbard L, Willcocks IR, O’Connell KS, Smith RL, et al. Genomic stratification of clozapine prescription patterns using schizophrenia polygenic scores. Biol Psychiatry. 2023;93:149–56.
Pardiñas AF, Holmans P, Pocklington AJ, Escott-Price V, Ripke S, Carrera N, et al. Common schizophrenia alleles are enriched in mutation-intolerant genes and in regions under strong background selection. Nat Genet. 2018;50:381–9.
Jönsson L, Simonsen J, Brain C, Kymes S, Watson L. Identifying and characterizing treatment-resistant schizophrenia in observational database studies. Int J Methods Psychiatr Res. 2019;28:e1778.
Bachmann CJ, Aagaard L, Bernardo M, Brandt L, Cartabia M, Clavenna A, et al. International trends in clozapine use: a study in 17 countries. Acta Psychiatr Scand. 2017;136:37–51.
Song J, Yao S, Kowalec K, Lu Y, Sariaslan A, Szatkiewicz JP, et al. The impact of educational attainment, intelligence and intellectual disability on schizophrenia: a Swedish population-based register and genetic study. Mol Psychiatry. 2022;27:2439–47.
Kendler KS, Ohlsson H, Fagan AA, Lichtenstein P, Sundquist J, Sundquist K. Academic achievement and drug abuse risk assessed using instrumental variable analysis and co-relative designs. JAMA Psychiatry. 2018;75:1182–8.
Acknowledgements
SUPER-Finland: We want to thank SUPER-Finland study participants. In addition, we want to thank the study nurses Panu Aunola, Marihelena Finne, Aino-Maija Haapasalo, Susanna Hotakainen, Ansa Järvinen, Veera Kemppainen, Kimmo Kontiainen, Maarit Kostiander, Eveliina Lehtinen, Maarit Lehtinen, Pia Maijala, Ulla Miettinen, Arja Minkkinen, Juha Mäkelä, Lea Nevalainen, Reijo Nevalainen, Paula Nurmi, Tero Rajatie, Outi Timonen, Kati Tuohimaa, Ulla Tyyni, Carita Vaittinen and Pia Virtanen, who worked on the field during the collection phase and the THL data collection and sample processing team whose work allowed for almost real-time surveillance of the collected material. Especially, we would like to thank Hannu Turunen for data management and Auli Toivola and Noora Ristiluoma for research coordination. We want to thank the outpatient policlinics and other healthcare units for their cooperation during the recruitment. We acknowledge the Genomics Platform of the Broad Institute of MIT and Harvard for genotyping the SUPER-Finland study samples. FinnGen: We want to acknowledge the participants and investigators of FinnGen study. The FinnGen project is funded by two grants from Business Finland (HUS 4685/31/2016 and UH 4386/31/2016) and the following industry partners: AbbVie Inc., AstraZeneca UK Ltd, Biogen MA Inc., Bristol Myers Squibb (and Celgene Corporation & Celgene International II Sàrl), Genentech Inc., Merck Sharp & Dohme LCC, Pfizer Inc., GlaxoSmithKline Intellectual Property Development Ltd, Sanofi US Services Inc., Maze Therapeutics Inc., Janssen Biotech Inc, Novartis AG, and Boehringer Ingelheim International GmbH. Following biobanks are acknowledged for delivering biobank samples to FinnGen: Auria Biobank (www.auria.fi/biopankki), THL Biobank (www.thl.fi/biobank), Helsinki Biobank (www.helsinginbiopankki.fi), Biobank Borealis of Northern Finland (https://www.ppshp.fi/Tutkimus-ja-opetus/Biopankki/Pages/Biobank-Borealis-briefly-in-English.aspx), Finnish Clinical Biobank Tampere (www.tays.fi/en-US/Research_and_development/Finnish_Clinical_Biobank_Tampere), Biobank of Eastern Finland (www.ita-suomenbiopankki.fi/en), Central Finland Biobank (www.ksshp.fi/fi-FI/Potilaalle/Biopankki), Finnish Red Cross Blood Service Biobank (www.veripalvelu.fi/verenluovutus/biopankkitoiminta), Terveystalo Biobank (www.terveystalo.com/fi/Yritystietoa/Terveystalo-Biopankki/Biopankki/) and Arctic Biobank (https://www.oulu.fi/en/university/faculties-and-units/faculty-medicine/northern-finland-birth-cohorts-and-arctic-biobank). All Finnish Biobanks are members of BBMRI.fi infrastructure (www.bbmri.fi). Finnish Biobank Cooperative -FINBB (https://finbb.fi/) is the coordinator of BBMRI-ERIC operations in Finland. The Finnish biobank data can be accessed through the Fingenious® services (https://site.fingenious.fi/en/) managed by FINBB.
Funding
The work was funded by the Sigrid Juselius Foundation and the Academy of Finland Center of Excellence for Complex Disease Genetics [grant numbers 312074, 336824, 352793] and supported by the Stanley Center for Psychiatric Research at Broad Institute (award/grant number is not applicable). The sequencing of the SUPER cohort was funded by the US National Institutes of Health Grants U54HG003067, 5U01MH105669 and 5UM1HG008895. We thank the Broad Institute Genomics Platform for genomic data generation efforts. AK was supported by The Swedish Society for Medical Research (Grant number: PD20-0190). OP was funded by Instrumentarium Science Foundation, Päivikki and Sakari Sohlberg Foundation, Jenny and Antti Wihuri Foundation. Open Access funding provided by University of Helsinki (including Helsinki University Central Hospital).
Author information
Authors and Affiliations
Consortia
Contributions
Study design & conceptualization: AK, JS, ML, MD, JT, KSK, AP and OP. Data collection and data curation: AK, JS, ML, TS, AAO, LU, WH, JH, EI, TJ, OK, TK, KL, JL, TM, TP, JNP, KS, ATH, JV, AW, MD, AP and OP. Developing the study analysis (including statistics): AK, JS, ML, LU, MD, JT, KSK, AP and OP. Supervision: MD, JT, KSK, AP and OP. Writing, reviewing and editing: All authors.
Corresponding author
Ethics declarations
Competing interests
AO is a shareholder and employee of Abomics, a company offering pharmacogenetics-related ICT-solutions and consultation services. MD is a member of the Pfizer Finland FinnGen Advisory Board and a founder of Maze Therapeutics.
Ethical approval
The study was approved by the Ethics Committee of the Hospital District of Helsinki and Uusimaa (Reference number 202/13/03/00/15). All participants were 18 years or older at the time of inclusion, and all have given written informed consent.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kämpe, A., Suvisaari, J., Lähteenvuo, M. et al. Genetic contribution to disease-course severity and progression in the SUPER-Finland study, a cohort of 10,403 individuals with psychotic disorders. Mol Psychiatry (2024). https://doi.org/10.1038/s41380-024-02516-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41380-024-02516-6