Abstract
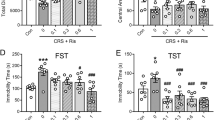
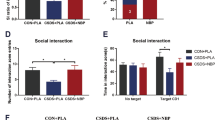
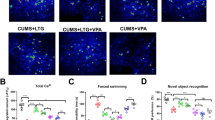
Major depressive disorder (MDD) is characterized by diverse debilitating symptoms that include loss of motivation and anhedonia. If multiple medications, psychotherapy, and electroconvulsive therapy fail in some patients with MDD, their condition is then termed treatment-resistant depression (TRD). MDD can be associated with abnormalities in the reward-system–dopaminergic mesolimbic pathway, in which the nucleus accumbens (NAc) and ventral tegmental area (VTA) play major roles. Deep brain stimulation (DBS) applied to the NAc alleviates the depressive symptoms of MDD. However, the mechanism underlying the effects of this DBS has remained elusive. In this study, using the chronic unpredictable mild stress (CUMS) mouse model, we investigated the behavioral and neurobiological effects of NAc-DBS on the multidimensional depression-like phenotypes induced by CUMS by integrating behavioral, in vivo microdialysis coupled with high-performance liquid chromatography–electrochemical detector (HPLC-ECD), calcium imaging, pharmacological, and genetic manipulation methods in freely moving mice. We found that long-term and repeated, but not single, NAc-DBS induced robust antidepressant responses in CUMS mice. Moreover, even a single trial NAc-DBS led to the elevation of the γ-aminobutyric acid (GABA) neurotransmitter, accompanied by the increase in dopamine (DA) neuron activity in the VTA. Both the inhibition of the GABAA receptor activity and knockdown of the GABAA-α1 gene in VTA-GABA neurons blocked the antidepressant effect of NAc-DBS in CUMS mice. Our results showed that NAc-DBS could disinhibit VTA-DA neurons by regulating the level of GABA and the activity of VTA-GABA in the VTA and could finally correct the depression-like behaviors in the CUMS mouse model.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data needed to evaluate the conclusions in the paper are present in the paper or the supplementary materials.
References
Friedman A, Frankel M, Flaumenhaft Y, Merenlender A, Pinhasov A, Feder Y, et al. Programmed acute electrical stimulation of ventral tegmental area alleviates depressive-like behavior. Neuropsychopharmacology. 2009;34:1057–66.
Orgnization WHO. Depressive disorder (depression). https://www.who.int/news-room/fact-sheets/detail/depression, 2023.
Al-Harbi KS. Treatment-resistant depression: therapeutic trends, challenges, and future directions. Patient Prefer Adherence. 2012;6:369–88.
Vieta E, Colom F. Therapeutic options in treatment-resistant depression. Ann Med. 2011;43:512–30.
Fostick L, Silberman A, Beckman M, Spivak B, Amital D. The economic impact of depression: resistance or severity? Eur Neuropsychopharmacol. 2010;20:671–5.
Luciano M, Del Vecchio V, Giacco D, De Rosa C, Malangone C, Fiorillo A. A ‘family affair’? The impact of family psychoeducational interventions on depression. Expert Rev Neurother. 2012;12:83–91.
Huang Y, Wang Y, Wang H, Liu Z, Yu X, Yan J, et al. Prevalence of mental disorders in China: a cross-sectional epidemiological study. Lancet Psychiatry. 2019;6:211–24.
Pitzer C, Kurpiers B, Eltokhi A. Sex differences in depression-like behaviors in adult mice depend on endophenotype and strain. Front Behav Neurosci. 2022;16:838122.
Schmuckermair C, Gaburro S, Sah A, Landgraf R, Sartori SB, Singewald N. Behavioral and neurobiological effects of deep brain stimulation in a mouse model of high anxiety- and depression-like behavior. Neuropsychopharmacology. 2013;38:1234–44.
Bewernick BH, Kayser S, Sturm V, Schlaepfer TE. Long-term effects of nucleus accumbens deep brain stimulation in treatment-resistant depression: evidence for sustained efficacy. Neuropsychopharmacology. 2012;37:1975–85.
Lozano AM, Giacobbe P, Hamani C, Rizvi SJ, Kennedy SH, Kolivakis TT, et al. A multicenter pilot study of subcallosal cingulate area deep brain stimulation for treatment-resistant depression. J Neurosurg. 2012;116:315–22.
Benabid AL, Pollak P, Louveau A, Henry S, de Rougemont J. Combined (thalamotomy and stimulation) stereotactic surgery of the VIM thalamic nucleus for bilateral Parkinson disease. Appl Neurophysiol. 1987;50:344–6.
Dougherty DD. Deep brain stimulation: clinical applications. Psychiatr Clin North Am. 2018;41:385–94.
Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26.
Tremblay LK, Naranjo CA, Graham SJ, Herrmann N, Mayberg HS, Hevenor S, et al. Functional neuroanatomical substrates of altered reward processing in major depressive disorder revealed by a dopaminergic probe. Arch Gen Psychiatry. 2005;62:1228–36.
Sesack SR, Grace AA. Cortico-basal ganglia reward network: microcircuitry. Neuropsychopharmacology. 2010;35:27–47.
Schlaepfer TE, Cohen MX, Frick C, Kosel M, Brodesser D, Axmacher N, et al. Deep brain stimulation to reward circuitry alleviates anhedonia in refractory major depression. Neuropsychopharmacology. 2008;33:368–77.
Gorwood P. Neurobiological mechanisms of anhedonia. Dialogues Clin Neurosci. 2008;10:291–9.
Nauczyciel C, Robic S, Dondaine T, Verin M, Robert G, Drapier D, et al. The nucleus accumbens: a target for deep brain stimulation in resistant major depressive disorder. J Mol Psychiatry. 2013;1:17.
Gershon AA, Vishne T, Grunhaus L. Dopamine D2-like receptors and the antidepressant response. Biol Psychiatry. 2007;61:145–53.
Kapur S, Mann JJ. Role of the dopaminergic system in depression. Biol Psychiatry. 1992;32:1–17.
Reddy PL, Khanna S, Subhash MN, Channabasavanna SM, Rao BS. CSF amine metabolites in depression. Biol Psychiatry. 1992;31:112–8.
Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35:192–216.
Edwards NJ, Tejeda HA, Pignatelli M, Zhang S, McDevitt RA, Wu J, et al. Circuit specificity in the inhibitory architecture of the VTA regulates cocaine-induced behavior. Nat Neurosci. 2017;20:438–48.
Naranjo CA, Tremblay LK, Busto UE. The role of the brain reward system in depression. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25:781–823.
Herrington TM, Cheng JJ, Eskandar EN. Mechanisms of deep brain stimulation. J Neurophysiol. 2016;115:19–38.
Benabid AL, Benazzous A, Pollak P. Mechanisms of deep brain stimulation. Mov Disord. 2002;17:S73–74.
Moser A, Gieselberg A, Ro B, Keller C, Qadri F. Deep brain stimulation: response to neuronal high frequency stimulation is mediated through GABA(A) receptor activation in rats. Neurosci Lett. 2003;341:57–60.
Yan N, Chen N, Zhu H, Zhang J, Sim M, Ma Y, et al. High-frequency stimulation of nucleus accumbens changes in dopaminergic reward circuit. PLoS One. 2013;8:e79318.
Zhu Z, Wang G, Ma K, Cui S, Wang JH. GABAergic neurons in nucleus accumbens are correlated to resilience and vulnerability to chronic stress for major depression. Oncotarget. 2017;8:35933–45.
Henny P, Brown MT, Northrop A, Faunes M, Ungless MA, Magill PJ, et al. Structural correlates of heterogeneous in vivo activity of midbrain dopaminergic neurons. Nat Neurosci. 2012;15:613–9.
Vitek JL. Mechanisms of deep brain stimulation: excitation or inhibition. Mov Disord. 2002;17:S69–72.
Elizalde N, Garcia-Garcia AL, Totterdell S, Gendive N, Venzala E, Ramirez MJ, et al. Sustained stress-induced changes in mice as a model for chronic depression. Psychopharmacology (Berl). 2010;210:393–406.
Chen Y, Cai W, Li C, Su Z, Guo Z, Li Z, et al. Sex differences in peripheral monoamine transmitter and related hormone levels in chronic stress mice with a depression-like phenotype. PeerJ. 2022;10:e14014.
Willner P, Muscat R, Papp M. Chronic mild stress-induced anhedonia: a realistic animal model of depression. Neurosci Biobehav Rev. 1992;16:525–34.
Duman CH. Models of depression. Vitam Horm. 2010;82:1–21.
Mutlu O, Gumuslu E, Ulak G, Celikyurt IK, Kokturk S, Kir HM, et al. Effects of fluoxetine, tianeptine and olanzapine on unpredictable chronic mild stress-induced depression-like behavior in mice. Life Sci. 2012;91:1252–62.
Goodwill HL, Manzano-Nieves G, Gallo M, Lee HI, Oyerinde E, Serre T, et al. Early life stress leads to sex differences in development of depressive-like outcomes in a mouse model. Neuropsychopharmacology. 2019;44:711–20.
Cryan JF, Mombereau C. In search of a depressed mouse: utility of models for studying depression-related behavior in genetically modified mice. Mol Psychiatry. 2004;9:326–57.
Cryan JF, Mombereau C, Vassout A. The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice. Neurosci Biobehav Rev. 2005;29:571–625.
Francis TC, Chandra R, Friend DM, Finkel E, Dayrit G, Miranda J, et al. Nucleus accumbens medium spiny neuron subtypes mediate depression-related outcomes to social defeat stress. Biol Psychiatry. 2015;77:212–22.
Gross M, Pinhasov A. Chronic mild stress in submissive mice: Marked polydipsia and social avoidance without hedonic deficit in the sucrose preference test. Behav Brain Res. 2016;298:25–34.
Liu MY, Yin CY, Zhu LJ, Zhu XH, Xu C, Luo CX, et al. Sucrose preference test for measurement of stress-induced anhedonia in mice. Nat Protoc. 2018;13:1686–98.
Yankelevitch-Yahav R, Franko M, Huly A, Doron R The forced swim test as a model of depressive-like behavior. J Vis Exp 2015.
Falowski SM, Sharan A, Reyes BA, Sikkema C, Szot P, Van Bockstaele EJ. An evaluation of neuroplasticity and behavior after deep brain stimulation of the nucleus accumbens in an animal model of depression. Neurosurgery. 2011;69:1281–90.
Engel M, Eggert C, Kaplick PM, Eder M, Roh S, Tietze L, et al. The Role of m(6)A/m-RNA methylation in stress response regulation. Neuron. 2018;99:389–403.e389.
Zingg B, Peng B, Huang J, Tao HW, Zhang LI. Synaptic specificity and application of anterograde transsynaptic AAV for probing neural circuitry. J Neurosci. 2020;40:3250–67.
McCracken CB, Grace AA. Nucleus accumbens deep brain stimulation produces region-specific alterations in local field potential oscillations and evoked responses in vivo. J Neurosci. 2009;29:5354–63.
Song N, Du J, Gao Y, Yang S. Epitranscriptome of the ventral tegmental area in a deep brain-stimulated chronic unpredictable mild stress mouse model. Transl Neurosci. 2020;11:402–18.
Tye KM, Mirzabekov JJ, Warden MR, Ferenczi EA, Tsai HC, Finkelstein J, et al. Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature. 2013;493:537–41.
Friedman AK, Walsh JJ, Juarez B, Ku SM, Chaudhury D, Wang J, et al. Enhancing depression mechanisms in midbrain dopamine neurons achieves homeostatic resilience. Science. 2014;344:313–9.
Okada H, Matsushita N, Kobayashi K, Kobayashi K. Identification of GABAA receptor subunit variants in midbrain dopaminergic neurons. J Neurochem. 2004;89:7–14.
Tan KR, Brown M, Labouebe G, Yvon C, Creton C, Fritschy JM, et al. Neural bases for addictive properties of benzodiazepines. Nature. 2010;463:769–74.
Perlmutter JS, Mink JW. Deep brain stimulation. Annu Rev Neurosci. 2006;29:229–57.
Miocinovic S, Somayajula S, Chitnis S, Vitek JL. History, applications, and mechanisms of deep brain stimulation. JAMA Neurol. 2013;70:163–71.
Bewernick BH, Hurlemann R, Matusch A, Kayser S, Grubert C, Hadrysiewicz B, et al. Nucleus accumbens deep brain stimulation decreases ratings of depression and anxiety in treatment-resistant depression. Biol Psychiatry. 2010;67:110–6.
Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–8.
Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404.
Russo SJ, Nestler EJ. The brain reward circuitry in mood disorders. Nat Rev Neurosci. 2013;14:609–25.
Lim BK, Huang KW, Grueter BA, Rothwell PE, Malenka RC. Anhedonia requires MC4R-mediated synaptic adaptations in nucleus accumbens. Nature. 2012;487:183–9.
Stuber GD, Sparta DR, Stamatakis AM, van Leeuwen WA, Hardjoprajitno JE, Cho S, et al. Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature. 2011;475:377–80.
Britt JP, Benaliouad F, McDevitt RA, Stuber GD, Wise RA, Bonci A. Synaptic and behavioral profile of multiple glutamatergic inputs to the nucleus accumbens. Neuron. 2012;76:790–803.
Nicola SM. The nucleus accumbens as part of a basal ganglia action selection circuit. Psychopharmacology (Berl). 2007;191:521–50.
Smith RJ, Lobo MK, Spencer S, Kalivas PW. Cocaine-induced adaptations in D1 and D2 accumbens projection neurons (a dichotomy not necessarily synonymous with direct and indirect pathways). Curr Opin Neurobiol. 2013;23:546–52.
Lenz JD, Lobo MK. Optogenetic insights into striatal function and behavior. Behav Brain Res. 2013;255:44–54.
Kravitz AV, Kreitzer AC. Striatal mechanisms underlying movement, reinforcement, and punishment. Physiology (Bethesda). 2012;27:167–77.
Maia TV, Frank MJ. From reinforcement learning models to psychiatric and neurological disorders. Nat Neurosci. 2011;14:154–62.
Lobo MK, Nestler EJ. The striatal balancing act in drug addiction: distinct roles of direct and indirect pathway medium spiny neurons. Front Neuroanat. 2011;5:41.
Freeze BS, Kravitz AV, Hammack N, Berke JD, Kreitzer AC. Control of basal ganglia output by direct and indirect pathway projection neurons. J Neurosci. 2013;33:18531–9.
Sah A, Schmuckermair C, Sartori SB, Gaburro S, Kandasamy M, Irschick R, et al. Anxiety- rather than depression-like behavior is associated with adult neurogenesis in a female mouse model of higher trait anxiety- and comorbid depression-like behavior. Transl Psychiatry. 2012;2:e171.
Barr LC, Heninger GR, Goodman W, Charney DS, Price LH. Effects of fluoxetine administration on mood response to tryptophan depletion in healthy subjects. Biol Psychiatry. 1997;41:949–54.
Gelfin Y, Gorfine M, Lerer B. Effect of clinical doses of fluoxetine on psychological variables in healthy volunteers. Am J Psychiatry. 1998;155:290–2.
Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129.
Ikemoto S. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res Rev. 2007;56:27–78.
Lammel S, Hetzel A, Hackel O, Jones I, Liss B, Roeper J. Unique properties of mesoprefrontal neurons within a dual mesocorticolimbic dopamine system. Neuron. 2008;57:760–73.
Ciccarelli A, Calza A, Panzanelli P, Concas A, Giustetto M, Sassoe-Pognetto M. Organization of GABAergic synaptic circuits in the rat ventral tegmental area. PLoS ONE. 2012;7:e46250.
Margolis EB, Toy B, Himmels P, Morales M, Fields HL. Identification of rat ventral tegmental area GABAergic neurons. PLoS ONE. 2012;7:e42365.
Nair-Roberts RG, Chatelain-Badie SD, Benson E, White-Cooper H, Bolam JP, Ungless MA. Stereological estimates of dopaminergic, GABAergic and glutamatergic neurons in the ventral tegmental area, substantia nigra and retrorubral field in the rat. Neuroscience. 2008;152:1024–31.
Johnson SW, North RA. Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci. 1992;12:483–8.
Bocklisch C, Pascoli V, Wong JC, House DR, Yvon C, de Roo M, et al. Cocaine disinhibits dopamine neurons by potentiation of GABA transmission in the ventral tegmental area. Science. 2013;341:1521–5.
Zhong P, Vickstrom CR, Liu X, Hu Y, Yu L, Yu HG, et al. HCN2 channels in the ventral tegmental area regulate behavioral responses to chronic stress. Elife. 2018;7:e32420.
Vega-Quiroga I, Yarur HE, Gysling K. Lateral septum stimulation disinhibits dopaminergic neurons in the antero-ventral region of the ventral tegmental area: Role of GABA-A alpha 1 receptors. Neuropharmacology. 2018;128:76–85.
Enz R. GABA(C) receptors: a molecular view. Biol Chem. 2001;382:1111–22.
Morales M, Margolis EB. Ventral tegmental area: cellular heterogeneity, connectivity and behaviour. Nat Rev Neurosci. 2017;18:73–85.
Omelchenko N, Sesack SR. Ultrastructural analysis of local collaterals of rat ventral tegmental area neurons: GABA phenotype and synapses onto dopamine and GABA cells. Synapse. 2009;63:895–906.
Watabe-Uchida M, Zhu L, Ogawa SK, Vamanrao A, Uchida N. Whole-brain mapping of direct inputs to midbrain dopamine neurons. Neuron. 2012;74:858–73.
Beier KT, Steinberg EE, DeLoach KE, Xie S, Miyamichi K, Schwarz L, et al. Circuit architecture of VTA dopamine neurons revealed by systematic input-output mapping. Cell. 2015;162:622–34.
Faget L, Osakada F, Duan J, Ressler R, Johnson AB, Proudfoot JA, et al. Afferent inputs to neurotransmitter-defined cell types in the ventral tegmental area. Cell Rep. 2016;15:2796–808.
Xi ZX, Stein EA. Nucleus accumbens dopamine release modulation by mesolimbic GABAA receptors-an in vivo electrochemical study. Brain Res. 1998;798:156–65.
van Zessen R, Phillips JL, Budygin EA, Stuber GD. Activation of VTA GABA neurons disrupts reward consumption. Neuron. 2012;73:1184–94.
Steffensen SC, Svingos AL, Pickel VM, Henriksen SJ. Electrophysiological characterization of GABAergic neurons in the ventral tegmental area. J Neurosci. 1998;18:8003–15.
Bespalov A, Steckler T, Altevogt B, Koustova E, Skolnick P, Deaver D, et al. Failed trials for central nervous system disorders do not necessarily invalidate preclinical models and drug targets. Nat Rev Drug Discov. 2016;15:516.
Nestler EJ, Hyman SE. Animal models of neuropsychiatric disorders. Nat Neurosci. 2010;13:1161–9.
Shemesh Y, Chen A. A paradigm shift in translational psychiatry through rodent neuroethology. Mol Psychiatry. 2023;28:993–1003.
Acknowledgements
We thank Academician Yizheng Wang and his team from the Center of Military Brain Science, Institute of Military Cognition and Brain Sciences, Academy of Military Medical Sciences for their support of the experiment.
Funding
The study was supported by the Beijing Natural Science Foundation (Grant No. 7244369).
Author information
Authors and Affiliations
Contributions
NS, SY, YG and ZL initiated and designed the research. NS wrote the manuscript. NS, SL, CY and ZL performed all experiments and analyzed and interpreted the results. All the authors approved the submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Song, N., Liu, Z., Gao, Y. et al. NAc-DBS corrects depression-like behaviors in CUMS mouse model via disinhibition of DA neurons in the VTA. Mol Psychiatry (2024). https://doi.org/10.1038/s41380-024-02476-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41380-024-02476-x