Abstract
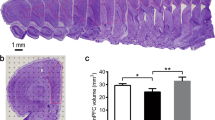
Running exercise has been shown to alleviate depressive symptoms. However, the mechanism underlying the antidepressant effects of running exercise is not fully understood. The imbalance of M1/M2 microglia phenotype/polarization and concomitant dysregulation of neuroinflammation play crucial roles in the pathogenesis of depression. Running exercise increases circulating levels of adiponectin which is known to cross the blood‒brain barrier and suppress inflammatory responses. AdipoR1 is an adiponectin receptor that is involved in regulating microglial phenotypes and activation states. However, whether running exercise regulates hippocampal microglial phenotypes and neuroinflammation through adiponectin/AdipoR1 to exert its antidepressant effects remains unclear. In the current study, 4 weeks of running exercise significantly alleviated the depressive-like behaviors of chronic unpredictable stress (CUS)-exposed mice. Moreover, running exercise decreased the microglial numbers and altered microglial morphology in three subregions of the hippocampus to restore the M1/M2 balance; these effects were accompanied by regulation of pro-/anti-inflammatory cytokine production and secretion in CUS-exposed mice. These effects may involve elevation of peripheral tissue (adipose tissue and muscle) and plasma adiponectin levels, and hippocampal AdipoR1 levels as well as activation of the AMPK-NF-κB/STAT3 signaling pathway by running exercise. When an adeno-associated virus was used to knock down hippocampal AdipoR1, mice showed depressive-like behaviors and alterations in microglia and inflammatory factor expression in the hippocampus that were similar to those observed in CUS-exposed mice. Together, these results suggest that running exercise maintains the M1/M2 balance and inhibits neuroinflammation in the hippocampus of CUS-exposed mice. These effects might occur via adiponectin/AdipoR1-mediated activation of the AMPK-NF-κB/STAT3 signaling pathway.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The datasets acquired for this study are available from the corresponding author upon reasonable request.
References
Smith K. Mental health: a world of depression. Nature. 2014;515:181.
Malhi GS, Mann JJ. Depression. Lancet. 2018;392:2299–312.
Rimer J, Dwan K, Lawlor DA, Greig CA, McMurdo M, Morley W, et al. Exercise for depression. Cochrane Database Syst Rev. 2012;7:CD004366.
Rethorst CD, Wipfli BM, Landers DM. The antidepressive effects of exercise: a meta-analysis of randomized trials. Sports Med. 2009;39:491–511.
Gujral S, Aizenstein H, Reynolds CF, Butters MA, Erickson KI. Exercise effects on depression: possible neural mechanisms. Gen Hosp Psychiatry. 2017;49:2–10.
Xiao K, Luo Y, Liang X, Tang J, Wang J, Xiao Q, et al. Beneficial effects of running exercise on hippocampal microglia and neuroinflammation in chronic unpredictable stress-induced depression model rats. Transl Psychiatry. 2021;11:461.
Tang J, Liang X, Dou X, Qi Y, Yang C, Luo Y, et al. Exercise rather than fluoxetine promotes oligodendrocyte differentiation and myelination in the hippocampus in a male mouse model of depression. Transl Psychiatry. 2021;11:622.
Li Y, Luo Y, Tang J, Liang X, Wang J, Xiao Q, et al. The positive effects of running exercise on hippocampal astrocytes in a rat model of depression. Transl Psychiatry. 2021;11:83.
Huang D, Xiao Q, Tang J, Liang X, Wang J, Hu M, et al. Positive effects of running exercise on astrocytes in the medial prefrontal cortex in an animal model of depression. J Comp Neurol. 2022;530:3056–71.
Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65:732–41.
Woodburn SC, Bollinger JL, Wohleb ES. The semantics of microglia activation: neuroinflammation, homeostasis, and stress. J Neuroinflammation. 2021;18:258.
Garaschuk O, Verkhratsky A. Physiology of microglia. Methods Mol Biol. 2019;2034:27–40.
Orihuela R, McPherson CA, Harry GJ. Microglial M1/M2 polarization and metabolic states. Br J Pharmacol. 2016;173:649–65.
Hu X, Leak RK, Shi Y, Suenaga J, Gao Y, Zheng P, et al. Microglial and macrophage polarization-new prospects for brain repair. Nat Rev Neurol. 2015;11:56–64.
Zhang L, Zhang J, You Z. Switching of the microglial activation phenotype is a possible treatment for depression disorder. Front Cell Neurosci. 2018;12:306.
Spielman LJ, Little JP, Klegeris A. Physical activity and exercise attenuate neuroinflammation in neurological diseases. Brain Res Bull. 2016;125:19–29.
Liu W, Ge T, Leng Y, Pan Z, Fan J, Yang W, et al. The role of neural plasticity in depression: from hippocampus to prefrontal cortex. Neural Plast. 2017;2017:6871089.
Fang H, Judd RL. Adiponectin regulation and function. Compr Physiol. 2018;8:1031–63.
Bloemer J, Pinky PD, Govindarajulu M, Hong H, Judd R, Amin RH, et al. Role of adiponectin in central nervous system disorders. Neural Plast. 2018;2018:4593530.
Leo R, Di Lorenzo G, Tesauro M, Cola C, Fortuna E, Zanasi M, et al. Decreased plasma adiponectin concentration in major depression. Neurosci Lett. 2006;407:211–3.
Narita K, Murata T, Takahashi T, Kosaka H, Omata N, Wada Y. Plasma levels of adiponectin and tumor necrosis factor-alpha in patients with remitted major depression receiving long-term maintenance antidepressant therapy. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:1159–62.
Yau SY, Li A, Hoo RLC, Ching YP, Christie BR, Lee TMC, et al. Physical exercise-induced hippocampal neurogenesis and antidepressant effects are mediated by the adipocyte hormone adiponectin. Proc Natl Acad Sci USA. 2014;111:15810–5.
Yamauchi T, Nio Y, Maki T, Kobayashi M, Takazawa T, Iwabu M, et al. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat Med. 2007;13:332–9.
Thundyil J, Pavlovski D, Sobey CG, Arumugam TV. Adiponectin receptor signalling in the brain. Br J Pharmacol. 2012;165:313–27.
Zhao L, Chen S, Sherchan P, Ding Y, Zhao W, Guo Z, et al. Recombinant CTRP9 administration attenuates neuroinflammation via activating adiponectin receptor 1 after intracerebral hemorrhage in mice. J Neuroinflammation. 2018;15:215.
Song J, Choi SM, Kim BC. Adiponectin regulates the polarization and function of microglia via ppar-gamma signaling under amyloid beta toxicity. Front Cell Neurosci. 2017;11:64.
Liu Y, Chewchuk S, Lavigne C, Brûlé S, Pilon G, Houde V, et al. Functional significance of skeletal muscle adiponectin production, changes in animal models of obesity and diabetes, and regulation by rosiglitazone treatment. Am J Physiol Endocrinol Metab. 2009;297:E657–E664.
Eyre H, Baune BT. Neuroimmunological effects of physical exercise in depression. Brain Behav Immun. 2012;26:251–66.
Kohler O, Benros ME, Nordentoft M, Farkouh ME, Iyengar RL, Mors O, et al. Effect of anti-inflammatory treatment on depression, depressive symptoms, and adverse effects: a systematic review and meta-analysis of randomized clinical trials. JAMA Psychiatry. 2014;71:1381–91.
Karperien A, Ahammer H, Jelinek HF. Quantitating the subtleties of microglial morphology with fractal analysis. Front Cell Neurosci. 2013;7:3.
Morrison H, Young K, Qureshi M, Rowe RK, Lifshitz J. Quantitative microglia analyses reveal diverse morphologic responses in the rat cortex after diffuse brain injury. Sci Rep. 2017;7:13211.
Zhang J, Xie X, Tang M, Zhang J, Zhang B, Zhao Q, et al. Salvianolic acid B promotes microglial M2-polarization and rescues neurogenesis in stress-exposed mice. Brain Behav Immun. 2017;66:111–24.
Zhang L, Tang M, Xie X, Zhao Q, Hu N, He H, et al. Ginsenoside Rb1 induces a pro-neurogenic microglial phenotype via PPARgamma activation in male mice exposed to chronic mild stress. J Neuroinflammation. 2021;18:171.
van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci USA. 1999;96:13427–31.
Gong H, Tai H, Huang N, Xiao P, Mo C, Wang X, et al. Nrf2-SHP cascade-mediated STAT3 inactivation contributes to AMPK-driven protection against endotoxic inflammation. Front Immunol. 2020;11:414.
Guo S, Wang H, Yin Y. Microglia polarization from M1 to M2 in neurodegenerative diseases. Front Aging Neurosci. 2022;14:815347.
Chen S, Dong Z, Cheng M, Zhao Y, Wang M, Sai N, et al. Homocysteine exaggerates microglia activation and neuroinflammation through microglia localized STAT3 overactivation following ischemic stroke. J Neuroinflammation. 2017;14:187.
Acknowledgements
The authors thank all the staff in the Laboratory Animal Center, Chongqing Medical University, People’s Republic of China, for providing assistance with the study.
Funding
This work was supported by National Natural Science Foundation of China (82171522, 82001436, 81871073, 82001435), Natural Science Foundation of Chongqing, China (cstc2021jcyj-msxmX0110), and Scientific and Technological Research Program of Chongqing Municipal Education Commission (KJQN202000402).
Author information
Authors and Affiliations
Contributions
LL participated in the design, research, and data analysis and wrote the draft manuscript. YML, JT, and XL guided the experiments and provided technical assistance. YYW provided technical assistance. YL, PLZ, MZ, LQ, YHD, JL, LJ, DJH, and QX conducted the animal experiments. YYW, MZ, YNZ, and SW assisted in the confocal fluorescence microscopy scans. YML reviewed and revised the manuscript. YML and YT helped with the experimental design, supervised the overall project, and revised the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, L., Tang, J., Liang, X. et al. Running exercise alleviates hippocampal neuroinflammation and shifts the balance of microglial M1/M2 polarization through adiponectin/AdipoR1 pathway activation in mice exposed to chronic unpredictable stress. Mol Psychiatry (2024). https://doi.org/10.1038/s41380-024-02464-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41380-024-02464-1