Abstract
Oxytocin plays an important role in modulating social recognition memory. However, the direct implication of oxytocin neurons of the paraventricular nucleus of the hypothalamus (PVH) and their downstream hypothalamic targets in regulating short- and long-term forms of social recognition memory has not been fully investigated. In this study, we employed a chemogenetic approach to target the activity of PVH oxytocin neurons in male rats and found that specific silencing of this neuronal population led to an impairment in short- and long-term social recognition memory. We combined viral-mediated fluorescent labeling of oxytocin neurons with immunohistochemical techniques and identified the supramammillary nucleus (SuM) of the hypothalamus as a target of PVH oxytocinergic axonal projections in rats. We used multiplex fluorescence in situ hybridization to label oxytocin receptors in the SuM and determined that they are predominantly expressed in glutamatergic neurons, including those that project to the CA2 region of the hippocampus. Finally, we used a highly selective oxytocin receptor antagonist in the SuM to examine the involvement of oxytocin signaling in modulating short- and long-term social recognition memory and found that it is necessary for the formation of both. This study discovered a previously undescribed role for the SuM in regulating social recognition memory via oxytocin signaling and reinforced the specific role of PVH oxytocin neurons in regulating this form of memory.
Similar content being viewed by others
Introduction
Social recognition memory (SRM) is a fundamental component of social behavior, which sub-serves everyday life interactions and is conserved across several species, including rodents [1,2,3]. A key feature of SRM is the ability of a species to acquire, remember, and recall identities of conspecifics. This facet of cognition serves to maintain and facilitate social organizational structures among conspecifics [4]. Deficits in social recognition are a core feature of several psychiatric disorders, including autism spectrum disorder (ASD) and schizophrenia [5,6,7]. As such, gaining insights into the brain circuitry that drives SRM is key to understanding the neurological basis of these disorders.
Several studies have identified the neuropeptide oxytocin (OXT) as a major modulator of SRM in rodents [8,9,10]. OXT is produced in the paraventricular, the supraoptic, and the accessory nuclei of the hypothalamus (PVH, SON, and AN, respectively). All these nuclei project predominantly to the posterior pituitary gland, where OXT is released into the bloodstream to modulate peripheral activities such as milk ejection during breast feeding and uterus contraction during parturition [11]. In addition, PVH-OXT neurons project to a wide range of cortical and limbic structures including the hippocampus, medial amygdala, and the lateral septum, all of which are characterized by high levels of oxytocin receptor (OXTR) expression [12] and are part of the “social recognition memory circuit” [13,14,15,16]. Collectively, this knowledge leads to the assumption that neuronal activity of PVH-OXT neurons is critical for SRM, which to date has not been directly examined.
OXT fibers and OXTR expression were previously identified in the supramammillary nucleus of the hypothalamus (SuM) [17,18,19,20], a caudal hypothalamic nucleus that is positioned superior to the mammillary body [21]. The SuM is known to regulate hippocampal theta oscillations [22, 23] and to be involved in arousal [24], REM sleep [25], lactation [17], reinforcement learning and motivation [26,27,28,29,30]. Recent work in mice has also demonstrated a role of the SuM in processing social novelty information [31]. Specifically, the SuM plays a role in relaying contextual and socially salient information to the hippocampus through anatomically segregated populations of projection neurons; SuM to hippocampal CA2 (SuM→CA2) projecting neurons process socially salient information, whereas SuM to dentate gyrus (SuM→DG) projecting neurons carry context-specific information. The abundance of OXT fibers and receptors in the SuM, along with its role in social novelty processing, suggest that OXT signaling in the SuM is likely to regulate SRM.
In the present study, we used targeted chemogenetic inhibition to examine the specific role of PVH-OXT neurons in SRM in male rats. We then conducted viral tracing and immunohistochemical staining to identify OXT projections fibers in the SuM and complemented those with in situ hybridization to examine the presence and distribution of OXTR within SuM neurons. Additionally, we combined retrograde labeling to identify CA2 projecting SuM neurons and further examined the distribution of OXTRs across this population of neurons. Finally, we used a highly specific OXTR antagonist to examine if OXTR signaling in the SuM is crucial for SRM.
Materials and methods
Animals
Male Sprague Dawley (SD) rats (Charles River, Wilmington, MA, USA) were used as test subjects for all experiments. 3–5 week-old male Wistar and Wistar Hannover rats (Charles River, USA) were used as stimuli for the social recognition memory experiments. All stereotaxic injections and cannula implantations were performed at the age of 8 weeks. Animals were housed in 2 s under a 12 h light/dark cycle at 22 ± 2 °C with food and water available ad libitum. All animal procedures were carried out in accordance with protocols approved by the Institutional Animal Care and Use Committee at the Icahn School of Medicine at Mount Sinai. Sample size was determined using G*Power [32] and was based on pilot study performed using DREADDs/OXTR antagonist on social recognition memory. The effect size was calculated based on repeated measures ANOVA with α = 0.05, β = 0.1 and power = 0.8.
Experimental design
To take advantage of the designer receptors activated by designer drugs (DREADD) system, we used a cross-over design wherein the same rat received either 0.9% saline or clozapine N Oxide (CNO)/OXTR antagonist across the testing paradigm (Figs. 1a, 6a). Detailed description of the design is in Supplementary Information. Since the same animals were being used as within-subject controls (saline vs CNO or saline vs OXTR antagonist), a priori condition was used wherein animals that failed to exhibit short or long-term SRM on saline treatment were not considered for further analysis. This was determined based on a threshold of 0.05 on the ratio of duration of investigation (RDI) index calculated as (Investigation TimeNovel – Investigation TimeFamiliar) / (Investigation TimeNovel + Investigation TimeFamiliar).
a A schematic showing the behavioral experimental design. Saline and CNO treatments were counterbalanced between test days, and short and long-term SRM were counterbalanced between cohorts. b A schematic of the experimental design for the short-term SRM. Saline or CNO was injected 30 min prior to the 1st encounter. c Top: A representative trace (from one rat per treatment during the 2nd encounter) following saline or CNO injection. Bottom: Heat maps representing investigation time across all rats during Novel or Familiar stimuli investigation following Saline or CNO. Each row represents one rat. d Total investigation time of the Novel vs. Familiar stimuli during the 2-ns encounter. Saline-injected rats showed a clear preference for Novel over Familiar stimuli, whereas same rats injected with CNO showed no preference for either stimuli (two-way repeated measures (RM) ANOVA), social preference (Familiar vs. Novel) × treatment (Saline vs. CNO) interaction (F1,26 = 9.11, **P = 0.0056, n = 14), effect of social preference (F1,26 = 35.07, ****P < 0.0001), and effect of treatment (F1,26 = 1.7, P = 0.203), post-hoc, Sidak multiple comparison test, Saline (Familiar vs. Novel, ****P < 0.0001) and CNO (Familiar vs. Novel, P = 0.38, ns). e Total investigation time of social stimuli during the 1st encounter. There was no significant difference in the total investigation time between Saline and CNO treatment groups during the 1st encounter (two-tailed paired Student’s t-test, t12 = 0.45, P = 0.65, ns). f A schematic of the experimental design for long-term SRM. Saline or CNO was injected 15 min prior the 1st encounter. g same as (c) but for long-term SRM. h Total investigation time of the Novel vs. Familiar stimuli during the 2nd encounter. Saline-treated animals showed a clear preference for Novel over Familiar stimuli, whereas same rats injected with CNO showed no preference for either stimuli (two-way RM ANOVA), social preference (Familiar vs. Novel) × treatment (Saline vs. CNO) interaction (F1,26 = 10.51,**P = 0.0032, n = 14), effect of social preference (F1,26 = 12.34, **P = 0.0016), and effect of treatment (F1,26 = 0.0005, P = 0.98). post-hoc, Sidak multiple comparison test, Saline (Familiar vs. Novel, ****P < 0.0001) and CNO (Familiar vs. Novel, P = 0.973, ns). i Investigation time of the social stimuli during the 1st encounter. There were no significant differences between saline and CNO injection groups during the 1st encounter (two-tailed paired Student’s t-test, t12 = 0.67, P = 0.51, ns).
Viral vectors
For specific silencing of OXT neurons, we used a validated virus (AAV1/2-OXTp-hM4DGi-mCherry), previously shown to reduce mean frequency of spikes under current injection (40pA) and input resistance of OXT neurons [33]. To control for the non-specific effects of CNO, we used a virus that lacks the DREADD backbone (AAV1/2-OXTp-mcherry). To identify OXT neuron projection fibers from the PVH or SON, we used an anterograde virus driven by an OXT promoter (AAV1/2-OXTp-Venus) [34]. All OXTp-driven viruses were produced and validated by Dr. Valery Grinevich’s laboratory at the Central Institute of Mental Health, University of Heidelberg, Germany. To identify SuM to CA2 projection neurons, we used a retrograde virus (AAV-Retrograde-Cre) and a cre-dependent virus (AAV9-DIO-Ef1a-eYFP).
Stereotaxic surgery
Animals were anesthetized with 4% isoflurane and maintained at 2% isoflurane and 2% oxygen (at a flow-rate of 2 L/min). An incision was made along the dorsal midline of the skull, bregma and lambda identified and a small burr hole (50 µm) was drilled. The virus (AAV1/2-OXTp-hM4Dgi-mCherry, AAV1/2-OXTp-Venus, or AAV1/2-OXTp-mCherry) was loaded into a 20 μl NanoFil syringe fitted with a 33gauge needle (World Precision Instruments Inc, USA). 270 nl was injected into the PVH (A-P −1.7 mm, M-L ± 0.3 mm, D-V 8.0 mm) at a 10°. For CA2 (A-P −3.5 mm, M-L ± 4.2 mm, D-V 3.3 mm) and SuM (A-P −4.5 mm, M-L ± 4.2 mm, D-V 8.9 mm, 15°), 0.05 μl or 0.07 μl, respectively. Following injection, the syringe was left in place for 10 min before being withdrawn and wound closed using wound clips (Stoelting Inc, USA). Rats received intraoperative subcutaneous fluids for hydration (Thermo Fisher Scientific, USA) and buprenorphine (0.05 mg/kg) as analgesia for 24–72 h. For cannulation, a guide cannula (7 mm, P1 Technologies Inc, USA) was implanted at a 15° (A-P −4.6 mm, M-L – 0 mm). Two bone screws (Stoelting Inc, Wood Dale, IL, USA) were implanted on the skull and secured using dental cement (Stoelting Inc, USA). A dummy cannula (7 mm) was left in place and OXTR antagonist/saline was delivered using an infusion cannula (9 mm).
Drugs
CNO dissolved in 0.05% DMSO and 0.9% saline or 0.9% saline (+0.05% DMSO) was injected intraperitoneally (i.p) using a 1 ml syringe (BD Biosciences, CA). For OXTR antagonist experiments, a stock (1 mg/ml) of OXTR antagonist (desGly-NH2-d(CH2)5[D-Tyr2,Thr4]OVT) was prepared in 0.9% saline. A working stock of 0.25 μg/μl was prepared on the day of the experiment and injected using a syringe pump (Amuza Inc, USA). A 5ul Hamilton syringe (Hamilton Company, USA) was connected to a plastic tubing on one end and an infusion cannula on the other. Saline/OXTR antagonist (0.25ug/ul) was loaded into the infusion cannula and a volume of 0.3 μl (75 ng total) [35, 36] was injected at a rate of 0.1ul/min.
Short and long-term discrimination task
Short and Long-term SRM were assessed using previously published paradigms, known as social discrimination tasks [14, 37, 38]. Briefly, the paradigms involve an initial encounter with a social stimulus followed by a short (30 min) or long (24 h) inter-trial interval after which the test animal is simultaneously exposed to the same stimulus (“Familiar”) as before and a novel stimulus (“Novel”). Social recognition is considered to have occurred when the test rat shows greater preference for the novel stimulus over the previously encountered stimulus [39]. Test and stimulus rats were habituated to handling and to the testing arena for 4 days before testing. To assess short-term SRM, rats were placed in the testing arena (50 × 50 × 40 cm). 30 min later, they received an i.p injection of either saline or CNO (8 mg/kg) and 30 min after, a juvenile rat of a different strain (3–5 week old, Wistar or Wistar Hannover strain) was placed in an enclosure, introduced into the testing arena for the test rat to investigate for 5 min (1st encounter). Following an inter-trial interval (ITI) of 30 min, the juvenile rat from the 1st encounter (“Familiar”) and a new juvenile rat (“Novel”) were placed in two small enclosures and introduced in two opposing corners of the testing arena for the test animal to investigate for 10 min (“2nd encounter”). The strain of the juvenile rats used for the first encounter were randomized such that each test rat interacted with a different strain across treatment sessions. To assess long-term-SRM, test rats received an either saline or CNO (8 mg/kg) and were placed in the testing arena. 15 min later, a juvenile rat of a different strain (3–5 week old, Wistar or Wistar Hannover strain) was placed in the testing arena for the test rat to freely interact and investigate for 1 h (1st encounter). After a 24 h ITI, the test rat was placed in the testing arena for 1 h for habituation followed by introduction of the “Familiar” and a “Novel” rat that were placed in two small enclosures and introduced in two opposing corners of the testing arena for 10 min (2nd encounter). Stimuli rats of different strains were used to enhance SRM acquisition, especially for long-term SRM [38]. In order to minimize the influence of spatial memory, the positioning of the stimuli rats during the 2nd encounter was always different from the 1st encounter (short-term SRM) and randomized between test subjects across paradigms.
Novel object recognition memory
Novel object recognition task was performed based on a previously established protocol [40]. Test rats were injected with 0.9% saline or CNO (8 mg/kg). 15 min later, they were allowed to interact for 3 min with two identical objects (Lego or Cone), placed on one side of the arena (“1st encounter”). After a 30 min ITI, test rats were introduced to one of the objects from the first encounter (“Familiar”) and a novel object (“Novel”) recorded for 3 min. The choice of objects was randomized across treatment groups.
Behavioral analysis
All behaviors were scored and quantified using TrackRodent, an open-source Matlab based automated tracking system that uses a body-based algorithm [41, 42]. The traces and heat maps were also obtained using the same system. The source code can be accessed on GitHub (https://github.com/shainetser/TrackRodent). Videos were de-identified in order to keep the experimenter blinded to the treatment groups while setting up the analysis.
Histology and immunohistochemistry
Protocols, antibodies, and concentrations are detailed in Supplementary Information.
RNAscope
Rat Oxtr [43], vglut2 (slc17a6) [44] and vgat1 (slc32a1) [44] probes were purchased from ACDBio. Fresh brains were collected by cervical decapitation and flash frozen in a slurry of isopentane and dry ice. Tissue was immediately sectioned at 15 µm, mounted on glass slides (SuperFrost Plus Microscope Slides, Fisher Scientific, USA) and frozen at −80 °C until the day of experiment. RNAscope was performed following the manufacturer’s protocol (RNAscope Multiplex Fluorescent Reagent Kit, ACDBio, USA). Briefly, tissue sections were thawed at RT for 10 min, fixed with 4% PFA for 15 min at 4 °C, and dehydrated with ethanol. They were then incubated in H2O2 for 10 min and a mix of Oxtr, vglut2, and vgat1 probes added and incubated for 2 h in a 40 °C oven (HybEZ II Hybridization System, ACDBio, USA). This was followed by an amplification step and then incubation with opal dyes (Akoya Biosciences, USA) 520, 570, and 690 to visualize the RNA transcripts.
Microscopy and image analysis
PVH sections (10–12) from OXT-hM4DGi-mcherry injected rats were imaged on a confocal microscope (Leica SP5 DMI, Leica Micro-Systems, USA) at the Microscopy and Advanced Bioimaging CoRE at the Icahn School of Medicine at Mount Sinai. Sections were imaged at 20× and Z stacks were acquired at step size of 1.0 µm and stacked images were exported to FIJI (ImageJ) and single plane images were generated using Z project (maximum intensity projection) [45]. Fluorescent in situ hybridization (RNAscope) images were acquired on a Zeiss AxioImager Z2M with ApoTome.2 at 10×, 40×, and 63× magnification. Images were imported into FIJI and a grid drawn over the acquired image. Individual neurons were counted grid by grid using the cell counter plugin on FIJI (n = 3 rats, 1 section/rat). DAB-stained sections were acquired using a bright field microscope (EVOS, Thermo Fisher Scientific, USA).
Statistical analysis
Statistical analysis was performed using GraphPad prism 9.0 software (GraphPad Prism, USA). Total investigation time between Familiar and Novel social stimuli were evaluated using a two-way repeated measures analysis of variance (RM ANOVA) to compare main effects of treatment (Saline vs. CNO or Saline vs. OXTR antagonist) and social preference (Familiar vs. Novel). Sidak’s multiple comparison test was used for post-hoc testing.
Reagents
Detailed information on reagents and resources are in supplementary information.
Results
Chemogenetic silencing of PVH-OXT neurons impairs short and long-SRM
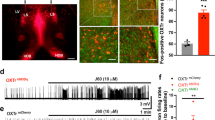
To test the direct role of PVH-OXT neurons in SRM, we examined if inhibition of PVH-OXT neuronal activity affects short and/or long-term social recognition memory following the experimental design in Fig. 1a. For this purpose, we used chemogenetic inhibitory DREADDs designed and validated to specifically express in OXT neurons and demonstrated to reduce mean frequency of spikes under current injection (40pA) and amplitude of OXT neurons (AAV1/2-OXTp-hM4Dgi-mCherry) [33]. We first confirmed and validated previous findings indicating the specific expression of the AAV1/2-OXTp-hM4DGi-mCherry virus in PVH-OXT neurons [33] (Supplementary Fig. 1a, b). Next, we tested the impact of chemogenetic silencing of PVH-OXT neurons on SRM, using the social discrimination task [14, 37]. When tested for short-term SRM (Fig. 1b), we found that rats injected with the inhibitory DREADDs showed a significant preference for the novel over the familiar social stimuli after saline (control) injection but failed to show a similar preference following CNO injection (Fig. 1c, d). Since CNO was administered prior to the initial interaction, we examined if this impaired the investigation time during the 1st encounter (time when the test rat interacts with the social stimulus for the first time, as shown in Fig. 1b). We compared the total investigation time following CNO and saline injection and found no difference between the two treatment conditions (Fig. 1e). Typically, SD rats engage in different lengths of bouts during the social discrimination task and show specific temporal dynamics that are distinct from other outbred rats and mice [46]. Additionally, bouts that are shorter than 6 s produce no clear separation of preference for the novel vs. familiar social stimuli, while bouts that are longer than 6 secs reflect more meaningful interactions in both mice [41] and rats [46]. Therefore, we further analyzed the data based on bout lengths. As expected, we found that during short bouts (≤6 s) rats did not show preference to the novel stimuli, regardless of the treatment (saline or CNO) (Supplementary Fig. 1c). During long bouts (≥6s), however, there was a significant preference for the novel over the familiar social stimuli following saline, but not CNO injection (Supplementary Fig. 1d).
To examine if the effects of PVH-OXT neural inhibition on social preference is consistent across the length of the social discrimination task, we also examined social preference as a function of time. We found that following saline injection, rats maintained their preference for the novel stimuli across time whereas following CNO injection, they showed no clear preference for either stimulus along time (Supplementary Fig. 1e, f). Furthermore, we ruled out any non-specific effect of CNO on short-term SRM by injecting an independent group of rats with a control virus that has the same backbone as the inhibitory DREADD virus, but lacks the hM4DGi receptor (AAV1/2-OXTp-mCherry) (Supplementary Fig. 2a–c). Finally, in order to confirm that the effect of PVH-OXT neuronal inhibition is specific for SRM and not to other aspects of non-social memory, we assessed a separate cohort of rats for their object recognition memory, using the novel object recognition memory task (Supplementary Fig. 2d). We found that OXTp-hM4DGi injected rats showed a clear preference for the novel over the familiar object, following saline or CNO injection (Supplementary Fig. 2e–g). Taken together, these results demonstrate that the activity of PVH-OXT neurons is necessary for short-term social recognition memory.
To determine if OXT neurons in the PVH are also necessary for long-term SRM we tested the same cohort of rats, which were tested on the short-term social discrimination task, on the long-term social discrimination task (Fig. 1f). We found that following saline injection, inhibitory DREADDs-injected rats showed significant preference for the novel over the familiar social stimuli. However, the same rats failed to show such preference following CNO injection (Fig. 1g, h). Furthermore, no significant differences between treatments were observed in the investigation time during the first 10 min of the 1st encounter (Fig. 1i). By classifying the stimuli interaction time into short (≤6 s) and long (≥6 s) bouts, we found that rats do not display any social preference when analyzing the short bout interactions, regardless of treatment (saline or CNO) (Supplementary Fig. 3a). However, preference to the novel stimuli over the familiar social stimuli was clearly observed when analyzing long bouts following saline, but not CNO injection (Supplemental data, Fig. 3b). By analyzing the social preference data across time, we found that preference to the novel stimuli was sustained throughout the duration of the testing period following saline but no clear preference was observed following CNO injection (Supplementary Fig. 3c, d). Altogether, these findings demonstrated that PVH-OXT neurons also play a critical role in long-term SRM and are likely to be a common substrate that mediates both short and long-term SRM.
The supramammillary nucleus is a target for OXT innervation that originates in the PVH but not the SON
The SuM is a caudal hypothalamic nuclei that is juxtaposed immediately over the mammillary bodies [23]. It has been shown to contain OXT fibers, yet the source of these fibers has not been determined [17]. Given the recently described role of the SuM in regulating social novelty processing in mice [31], we hypothesized that OXT action within the SuM is likely to contribute to the SuM’s role in social novelty processing and the formation of social memory. We first wanted to confirm previous findings by demonstrating the presence of OXT fibers in the SuM and determining the source of its innervation. For this purpose, we used immunohistochemistry with anti-OXT antibodies to visualize OXT fibers in the SuM and found that they are broadly distributed across the rostro-caudal parts of the SuM with fibers identified in both the medial (SuMm) and lateral part of the SuM (SuMl) (Fig. 2a, Supplementary Fig. 4). To determine the origin of these OXT fibers, we unilaterally injected, into the PVH or SON, an anterograde virus, which expresses Venus fluorescent protein specifically in OXT neurons (AAV1/2-OXTp-Venus), thus allowing specific labeling of OXT neurons and their projections [34]. We found that the PVH is a major source for OXT fibers in both the SuMm and SuMl (Fig. 2b, Supplementary Fig. 4) and observed punctate labeling as well as branched axons indicative of local innervation (Fig. 2a, b, Right, insets). We further confirmed the presence of OXT axonal terminals in the SuM by injecting an anterograde AAV-OXTp-synaptophysin-GFP virus [34], which expresses GFP fused to synaptophysin, a presynaptic terminal marker in OXT neurons (Supplementary Fig. 5a). Calretinin and parvalbumin have been used to distinguish the SuM, which is calretinin positive but parvalbumin negative, from the mammillary body, which is located caudal to the SuM and is parvalbumin positive but calretinin negative [23]. We, therefore, used these markers in combination with anti-OXT antibodies (Supplementary, Fig. 5b) or AAV1/2-OXTp-Venus (Supplemental data. Figure 5c) to confirm the high abundance of OXT fibers in the SuM, but not the mammillary body. Importantly, we did not detect any OXT fibers in the SuM when the virus (AAV1/2-OXTp-Venus) was injected into the SON (Fig. 2c), suggesting that the SON OXT neurons do not project to the SuM. All together, these experiments demonstrate that PVH-OXT neurons are a major source for OXT projection fibers in the SuM.
a Immunohistochemical staining for OXT in the PVH and SuM. Left: A representative PVH section stained with specific anti-OXT antibodies and developed using diaminobenzidiene (DAB) based enzymatic staining. Middle: A representative SuM section from the same animal as above was stained for cresyl violet to highlight anatomical structures. Right: An immediately adjacent SuM section to that in the middle image stained with anti-OXT antibody to highlight OXT fibers in the SuMm and the SuMl (4×). Inset shows a higher magnification (40×) image of the SuMm (highlighted by red dotted box). Black arrows point to axonal varicosities and branching axons. b Immunohistochemical staining for Venus in the PVH and SuM of rats injected with the AAV1/2-OXTp-Venus in the PVH. Left: A representative image of the PVH injected unilaterally with the AAV1/2-OXTp-Venus. Venus was identified using anti-GFP antibody and developed enzymatically using DAB based staining. Middle: Cresyl violet staining of the SuM to highlight the SuMl and SUMm. Right: An immediately adjacent SuM section to that in the middle image shows Venus-positive fibers distributed across the SuMm and SuMl (4×). Inset shows a higher magnification (40×) image of the SuMm (highlighted by red box). Black arrows point to axonal varicosities and branching axons. c Immunohistochemical staining for Venus in SON and SuM from rats injected with AAV1/2-OXTp-Venus in the SON. Left: AAV1/2-OXTp-Venus injected into the SON identified using anti-GFP antibody. Middle: Cresyl violet staining of SON injected group to highlight anatomical structures in the SuM. Right: An immediately adjacent SuM section to that in the middle image showing absence of Venus-positive fibers across the SuMm and SuMl (4×). −4.5 mm denotes position of the section relative to bregma. 3 V, 3rd ventricle, PVH, paraventricular nucleus of the hypothalamus. SuMm, medial supramammillary nucleus, SuMl, lateral supramammillary nucleus, MNu, Mammillary nucleus, pm, principal mammillary tract. Scale bar 100 µm.
OXT receptors are expressed by specific population of SuM neurons
After establishing that PVH-OXT are a major source of OXT innervation in the SuM, we set to determine if SuM neurons express OXTRs. Although previous studies have identified that the SuM contains OXTR using autoradiographic techniques in rats [19, 20] or transgenic mice [18], those studies were limited by their inability to determine the cellular distribution of the receptors. The SuM is comprised mostly of glutamatergic neurons and to a lesser extent of GABAergic, dopaminergic [47], substance P [48], and CCK-positive neurons [49]. Additionally, the SuM is one of the few brain regions in the rat where some neurons co-express both glutamate and GABA [44]. In order to determine the cellular distribution of OXTR within the SuM, we employed RNAscope, an in situ RNA hybridization (ISHr) technology, to identify OXTR transcripts (Oxtr) and examine their overlap with glutamatergic and GABAergic neurons. We used the vesicular glutamate transporter (vglut2), as a marker for excitatory neurons, and the vesicular GABA transporter (vgat1) as a marker for inhibitory neurons (Supplementary Fig. 6a). We first examined the proportion of SuM neurons that are GABAergic, glutamatergic, or both and then determined if Oxtr differentially segregate across these neural populations. We found that neurons within the SuM are primarily positive for vglut2 (83 ± 0.7%, # of vglut2+ neurons, 344 ± 2.3, total number of neurons, 413.6 ± 2.72; n = 3 rats, 1 section/rat), with a small population being positive for both vglut2 and vgat1 (10.7 ± 0.7%, # of vglut2+:vgat1+ neurons, 44.6 ± 3.17), and an even smaller population of neurons that were positive only for vgat1 (6 ± 0.8%, # of vgat1+ neurons, 25 ± 3.4) (Fig. 3a–c). These results were consistent with previously reported distribution of excitatory and inhibitory neurons in the SuM [44]. In order to determine how Oxtr segregates into these populations, we quantified the number of neurons that express Oxtr within each population. We found that nearly 60% of vglut2+ neurons are also Oxtr+ (# of vglut2+/oxtr+, 211 ± 25.1), whereas 48% of vglut2+:vgat1+ neurons are Oxtr+ (# of vglut2+:vgat1+/Oxtr+ neurons, 21.6 ± 1.2) and only 13% of vgat1+ are Oxtr+ # of vgat1+/Oxtr+ neurons, 3.3 ± 0.8 (Fig. 3d). These results indicate that OXTRs in the SuM are predominantly expressed in glutamatergic neurons and neurons that co-express glutamate, and GABA and in a very minor population of GABA neurons.
a RNAscope was performed on SuM tissue using probes for Oxtr, vglut2 (marker for glutamatergic neurons) and vgat1 (marker for GABAergic neurons). Left: Lower magnification (10×) shows Oxtr along with vglut2 and vgat1 expression in the SuM. Right: Higher magnification (40×) of SuM tissue shows distribution of Oxtr across vglut2+ and vgat1+ neurons. b Higher magnification (63×) of SuM tissue showing Oxtr localization in vglut2+ neurons (Left), vgat1+ neurons (Middle), or vglut2+:vgat1+ neurons (Right), highlighted by white arrows. c Quantification of vglut2+, vgat1+, and vglut2+:vgat1+ neurons in the SuM. d Quantification of Oxtr distribution across vglut2+ (Left), vgat1+ (Middle), and vglut2+:vgat1+ neurons (Right) neurons. n = 3, 1 section per animal. Scale bar 100 µm, SuM, Supramammillary nucleus. MNu, Mammillary nucleus, pm, principal mammillary tract. Data presented as Mean ± SEM.
SuMvglut2+ neurons that project to the hippocampal CA2 area co-express OXTR
SuM neurons have been shown to project to the hippocampus, specifically the hippocampal CA2 in mice [31, 50]. Here, we set to confirm if SuM→CA2 projections also exist in rats and to determine if OXTRs are specifically expressed in these projecting neurons. To this end, we injected a retrograde virus (AAV-Ef1α-Rg-Cre) into the hippocampal CA2 and a Cre-dependent reporter virus (AAV-Ef1α-DIO-eYFP) into the SuM (Fig. 4a). The AAV-Ef1a-DIO-eYFP virus travels anterogradely by virtue of its packaging capsid (AAV9) (Fig. 4a), thus the combination of the two viruses allowed us not only to identify CA2 projecting neurons in the SuM, but also to visualize axonal terminals of these neurons in the CA2 (Fig. 4b, c). We identified SuM→CA2 projections neurons across the rostro-caudal and dorso-ventral axis of spread (bregma, −4.3 to −4.6 mm) and in both the medial and the lateral boundary of the medial and the lateral aspect of the SuM (Fig. 4d). In order to determine if OXTRs are expressed by this subset of neurons, we performed ISHr for Oxtr and vglut2 or vgat1 and stained for GFP using immunohistochemistry and found Oxtr transcripts to be co-localized in SuMvglut2+/eYFP+→CA2 projecting neurons (Fig. 5a), but not on SuMvgat1+/eYFP+ neurons (Fig. 5b). Our quantification revealed that of the SuMvglut2+/eYFP+ (8.03 ± 1.12%) (# of vglut2+/eYFP+, 62 ± 2.13) neurons, 82.3 ± 12.8% (# of Oxtr+/vglut2+/eYFP+, 46 ± 4.5) express Oxtr transcripts, whereas none of the SuMvgat1+/eYFP+ (4.7 ± 0.93%) (# of vgat1+/eYFP+, 33.5 ± 1.08) contained Oxtr transcripts. Taken together, these results indicate that OXTRs are localized on excitatory SuM neurons that project to the hippocampal CA2 and suggest that activity of this neuronal population could be modulated by OXTR signaling.
a A schematic of the viral strategy to target and identify SuM→CA2 projecting neurons. A retrograde virus expressing a cre-recombinase (AAV-Ef1α-Rg-Cre) was injected into the hippocampal CA2 and a cre-dependent anterograde virus (AAV9-EF1ɑ-DIO-eYFP) was injected into the SuM. b A coronal section show SuM axonal terminals (labeled with eYFP) in the CA2 neurons (identified by anti-PCP4 staining). c A higher magnification of the hippocampal CA2. d Four representative images spanning the SuM show SuM→CA2 projecting neurons across the rostro-causal and dorso-ventral axis of the SuM. Calretinin was used to highlight the boundaries between the SuM and the mammillary nucleus. −4.3 to −4.6 mm denotes position of the section relative to bregma. Ge, Nucleus of Gemini, mt, mamillothalamic tract, pm, principal mammillary tract, SuMl, supramammillary nucleus lateral, SuMm, supramammillary nucleus medial. MNu, Mammillary nucleus. Scale bar: 5b and 5d, 500 µm; 5c, 100 µm.
a RNAscope using probes for vglut2 and Oxtr, followed by immunohistochemistry for eYFP (using anti-GFP antibody) on tissue section from rats injected in the hippocampal CA2 with a retrograde virus expressing a cre-recombinase (AAV-Ef1α-Rg-Cre) and in the SuM with a cre-dependent anterograde virus (AAV9-EF1ɑ-DIO-eYFP). Top: lower magnification of SuM showing expression of eYFP, vglut2, and Oxtr in the SuM. Bottom: higher magnification of the same Top images. White arrow point to the overlap between SuM→CA2 projecting neurons (labeled with eYFP), vglut2 and Oxtr. b same as in (a), but using the vgat1 instead of the vglut2 probe. White arrows point to the lack of expression of Oxtr in eYFP and vgat1+ neurons. c Quantification of the proportion of vglut2+ or vgat1+ neurons projecting to the CA2 region. d Quantification of Oxtr distribution across SuM-vglut2+→CA2 projecting neurons (Left) and SuM-vgat1+→CA2 projecting neurons (Right). n = 3, 1–3 sections per animal. Scale bar: 6a and 6b - Top panels, 250 µm. 6a, and 6b - Bottom panels, 50 µm.
Blocking OXTR in the SuM affects both short and long social recognition memory
To follow up on our findings, which indicated that the SuM is heavily innervated by PVH-OXT fibers and exhibits high expression of OXTR, we set to test if OXT downstream signaling within the SuM is necessary for SRM. To this end, we implanted a cannula to target the SuM (Supplementary Fig. 7) and infused a selective OXTR antagonist (0.3 μl, 75 ng total) [35, 36], 10 min before testing the rats on the short or long-term social discrimination tasks, using a cross-over design (Fig. 6a). We found that on the short-term SRM task (Fig. 6b), following saline infusion, subject rats showed a clear preference for the novel over the familiar social stimuli, whereas infusion of OXTR antagonist led to a lack of preference for either stimuli (Fig. 6c, d). Furthermore, we found no significant differences in investigation time between the saline and OXTR antagonist-infused rats during the 1st encounter (Fig. 6e). As before, when the short bouts were assessed, neither saline nor OXTR antagonist-injected rats showed any preference for the novel stimuli (Supplementary Fig. 8a). However, when bouts that were longer than 6 secs where assessed, saline-injected rats showed a clear and significant preference for the novel over the familiar stimuli, with no such preference observed following OXTR antagonist infusion (Supplementary Fig. 8b). The preference for the novel stimuli over the familiar stimuli in the saline-infused rats and the lack of clear preference in the OXTR-infused rats were sustained across time (Supplemental data, Fig. 8c, d). Overall, these results suggest that OXT signaling in the SuM is necessary for modulating short-term SRM.
a A schematic showing the behavioral experimental design. Saline and OXTR antagonist treatment were counterbalanced between test days, and short and long-term SRM was counterbalanced between cohorts. b A schematic of the behavioral paradigm for short-term SRM. Saline or OXTR antagonist was infused 10 min prior to the 1st encounter. c Top: A representative trace from one animal per treatment during the 2nd encounter following Saline or OXTR antagonist infusion. Bottom: Heat maps representing investigation time of all rats during novel or familiar investigation following saline or OXTR antagonist infusion. d Total investigation time of the novel vs. familiar stimuli during the 2nd encounter. Saline-infused rats showed a clear preference for Novel over Familiar stimuli, whereas the same rats showed no preference for Novel or Familiar stimuli after OXTR antagonist infusion (two-way RM ANOVA), social preference (Familiar vs. Novel) × treatment (Saline vs. OXTR antagonist) interaction (F1, 16 = 9.25, **P = 0.007, n = 9), effect of social preference (F1, 16 = 4.02, P = 0.06), and effect of treatment (F1, 16 = 0.01, P = 0.89). Post-hoc, Sidak multiple comparison test, Saline (Familiar vs. Novel) **P = 0.005, OXTR antagonist (Familiar vs. Novel, P = 0.96, ns). e Investigation time of the social stimuli during the 1st encounter. There was no significant difference between saline and OXTR antagonist treatment during the 1st encounter (two-tailed Student’s t-test, t5 = 1.513, P = 0.19, ns). f A schematic of the behavioral paradigm for long-term SRM. Saline or OXTR antagonist was infused 10 min prior to the 1st encounter. g Top: same as (c) but for long-term SRM. h Total investigation time of the novel vs. familiar stimuli during the 2nd encounter. Saline-infused rats showed a clear preference for novel over familiar stimuli, whereas the same rats showed no preference for the either stimuli after OXTR antagonist infusion (Two-way RM ANOVA, social preference × treatment (Saline v OXTR antagonist) interaction, F1,20 = 15.32, ***P = 0.0009, n = 11), effect of social preference (F1,20 = 15.27, ***P = 0.0009), and effect of treatment (F1,20 = 1.723, P = 0.2). Post-hoc, Sidak multiple comparison test, Saline (Familiar vs. Novel, ****P < 0.0001) and OXTR antagonist (Familiar vs. Novel, P = 0.78, ns). i There was no significant difference between saline and OXTR antagonist-infused group during the 1st encounter on the long-term SRM (two-tailed Student’s t-test, t10 = 0.24, P = 0.80, ns).
Similarly, we examined the impact of the OXTR antagonist on long-term SRM (Fig. 6f). We found that compared to saline infusion, where rats showed a clear preference for the novel over the familiar stimuli, rats infused with OXTR antagonist in the SuM showed a robust impairment in long-term SRM (Fig. 6g, h). Finally, there was no significant difference in the investigation time during the first 10 min of the 1st encounter between the saline and OXTR-infused groups (Fig. 6i). As expected, short interaction bouts showed no significant differences in the preference for the novel over the familiar social stimuli, regardless of whether rats were infused with saline or OXTR antagonist (Supplementary Fig. 8f). When long interaction bouts were assessed, saline-infused rats showed a clear preference for the novel over the familiar stimuli, whereas OXTR antagonist-infused rats failed to show a preference for either stimuli (Supplementary Fig. 8g). The preference for the novel stimuli over the familiar stimuli in the saline-infused rats and the lack of clear preference in the OXTR-infused rats were both sustained over time (Supplementary Fig. 8h, i).
Discussion
Social recognition memory is a key component of social behavior that is essential for distinguishing between familiar and novel conspecifics [3, 51] and is regulated by a defined brain circuit [2, 3] This circuit engages several neural substrates including the lateral and medial septum, prefrontal cortex, medial amygdala and hippocampus [14, 52,53,54]. Information processing within neural circuits is not hard-wired but rather adaptive to the surrounding environment, in part due to the activity of neuromodulators such as OXT [55,56,57].
In this study, we showed that acute silencing of OXT neurons within the PVH impaired the ability of rats to discriminate between novel and familiar social stimuli, indicating that activity of PVH-OXT neurons is critical for mediating SRM in rats. These findings align with the previously established role of OXT in short and long-term SRM [2, 8, 10, 15, 37, 52, 58] yet attributes, for the first time, a specific role for PVH-OXT in both forms of memory. In contrast to a recent study in male mice showing that chemogenetic silencing of PVH-OXT neurons decreases social investigation of a first-time presented (novel) social stimulus [59], our study further demonstrated that acute chemogenetic silencing of PVH-OXT neural activity in rats had no impact on social investigation of a novel social stimulus. Our findings align with discoveries from previous studies, which assessed social investigation and SRM in male mice and prairie voles that lacked the OXT or OXTR encoding gene [9, 10, 60]. Male OXT-KO or OXTR-KO mice and their WT and littermates spent similar amount of time investigating a first-time presented stimulus in a free interaction setup, indicating an intact approach and interest to engage in social interaction. However, when exposed repeatedly to the same stimulus, OXT-KO and the OXTR-KO mice failed to form SRM [9, 10]. In prairie voles, OXTR-KO males showed no deficits in investigating a novel stimulus on the three-chamber test, but showed deficits in SRM when presented with a novel and familiar stimulus [60]. These studies, together with our findings, suggest that OXT may not be essential for the act of social interaction per se, but rather necessary for the formation of SRM, which involves processing of sensory information, encoding the salience of social stimuli, and forming social memory. Notably, a recent study in female rats showed that specific silencing of the parvocellular population of PVH-OXT neurons led to a decrease in the investigation time of a social stimulus, but only during a free and not a contained social interaction [61]. Further investigation of these findings led the authors to conclude that social touch promotes social communication in female rats through the activity of the parvocellular PVH-OXT neurons. These findings raise an important question regarding the role of OXT and social touch, as well as other sensory modalities in salience encoding during social interaction and the formation of SRM, which ought to be addressed in future studies. The direct implication of PVH-OXT neurons in modulating social memory is of significance as rodent models with mutations in high-risk genes for autism spectrum disorder (ASD), have shown changes in the overall number of PVH-OXT neurons and/or reduced OXT levels, thus suggesting that modified OXT activity could underlie some of the social behavioral phenotype reported in these models [59, 62, 63]. Our own work in a rat model that harbors a mutation in a high-risk gene for ASD, Shank3, identified long- but not short-term SRM deficits that could be ameliorated with exogenous administration of OXT [37], thus reinforcing the need to dissect the role of OXT in modulating SRM.
The SuM is involved in several functions including arousal [24], REM sleep [25], lactation [17], locomotion [64], reinforcement learning, and motivation [26,27,28,29,30]. SuM activity is also important for synchronizing the frequency of theta activity in the hippocampus, which modulates spike-time coordination during spatial navigation to regulate spatial memory [23, 64,65,66]. Recently, Chen et al. demonstrated that the SuM is involved in processing social novelty information [31] via a specific pathway that connects the SuM with the hippocampal CA2 (SuM→CA2 pathway). They also showed that activation of the SuM→CA2 pathway regulates the excitation vs. inhibition (E/I) ratio within the CA2, which in turn could play a role in tuning the response of CA2 neurons to novel social stimuli. Although OXT fibers have been previously reported in the SuM [17], it was unclear if these fibers originated from the PVH or the SON. Our findings demonstrate that PVH-OXT neurons are a source for OXT fibers in the SuM. Furthermore, the SuM has been shown to express OXTRs in both mice [18] and rats [19, 20], however, to our knowledge, our study is the first to show that OXTRs is localized on glutamate, GABA, and glutamate/GABA co-expressing SuM neurons and that OXT receptors are expressed on glutamatergic neurons that project to the hippocampal CA2. These findings are significant considering the role of the SuM in regulating social novelty processing via the SuM→CA2 pathway [31], and together with our findings, they suggested that social memory is likely to be modulated by OXT signaling in the SuM. Indeed, we found that blocking OXT signaling within the SuM impaired both short- and long-term social memory, thus providing evidence for OXT’s role within the SuM in social memory.
Based on these findings and the accumulated knowledge in the field, we propose a working model where the PVH, the SuM, and the hippocampal CA2 work together to modulate SRM. Specifically, we propose that the PVH-OXT→SuM pathway acts to amplify the salience of the social stimulus via OXTR signaling within the SuM, while the SuM→CA2 pathway routes the social information to the CA2 to facilitate social memory. Future studies, designed to manipulate PVH-OXT→SuM or the SuMvglut2+/OXTR+→CA2 pathway at different time points during the SRM task, will narrow down the specific contribution of each of these pathways to the acquisition, consolidation, and/or recall phase of SRM. Furthermore, studies, using in vivo and in vitro recordings in the SuM following manipulation of PVH-OXT neuronal activity or OXTR agonist/antagonist administration will shed a light onto the molecular and cellular mechanisms by which OXT exerts its effect on SuM neurons and indirectly on CA2 neurons to regulate SRM. To the best of our knowledge, Cumbers and colleagues were the only to examine the effect of OXT on SuM neuronal activity [17]. They found that OXT infusion into the SuM increased the spiking rate of a subpopulation of SuM neurons and facilitated suckling-evoked SuM neural responses in lactating rats. Building on these findings, future in vitro studies should examine the effect of OXT not only on spontaneous firing, but also intrinsic excitability, as well as inhibitory and excitability synaptic transmission in SuM neurons, which will enhance our understanding of the OXT action in the SuM. In vivo studies, on the other hand, could examine the role of OXT in regulating SuM and CA2 neuronal activity during SRM and test the causality between OXT signaling, SuM and CA2 neuronal activity, and SRM.
Testing our working model will not only enhance our understanding of social brain circuits, but also has the potential to identify new targets for treatment of social behavior deficits, including deficits in SRM, which are pervasive in several psychiatric disorders such as schizophrenia and ASD [5,6,7].
Data availability
All the data generated for this manuscript have been included in this published article.
References
Penn, DJ, and Frommen, JG. Kin recognition: an overview of conceptual issues, mechanisms and evolutionary theory. In: Animal behaviour: evolution and mechanisms; 2010. p. 55–85. https://doi.org/10.1007/978-3-642-02624-9_3
Bielsky IF, Young LJ. Oxytocin, vasopressin, and social recognition in mammals. Peptides. 2004;25:1565–74. https://doi.org/10.1016/j.peptides.2004.05.019
Ferguson JN, Young LJ, Insel TR. The neuroendocrine basis of social recognition. Front Neuroendocrinol. 2002;23:200–24. https://doi.org/10.1006/frne.2002.0229
Insel TR, Fernald RD. How the brain processes social information: Searching for the social brain. Annu Rev Neurosci. 2004;27:697–722. https://doi.org/10.1146/annurev.neuro.27.070203.144148
Hoertnagl CM, Hofer A. Social cognition in serious mental illness. Curr Opin Psychiatry. 2014;27:197–202. https://doi.org/10.1097/YCO.0000000000000055
Oliver LD, Moxon-Emre I, Lai MC, Grennan L, Voineskos AN, Ameis SH. Social cognitive performance in schizophrenia spectrum disorders compared with autism spectrum disorder: a systematic review, meta-analysis, and meta-regression. JAMA Psychiatry. 2021;78:281–92. https://doi.org/10.1001/jamapsychiatry.2020.3908
Velikonja T, Fett AK, Velthorst E. Patterns of nonsocial and social cognitive functioning in adults with autism spectrum disorder: a systematic review and meta-analysis. JAMA Psychiatry. 2019;76:135–51. https://doi.org/10.1001/jamapsychiatry.2018.3645
Popik P, Vetulani J, van Ree JM. Low doses of oxytocin facilitate social recognition in rats. Psychopharmacol. 1992;106:71–74. https://doi.org/10.1007/BF02253591
Takayanagi Y, Yoshida M, Bielsky IF, Ross HE, Kawamata M, Onaka T, et al. Pervasive social deficits, but normal parturition, in oxytocin receptor-deficient mice. Proc Natl Acad Sci USA. 2005;102:16096–101. https://doi.org/10.1073/pnas.0505312102
Ferguson JN, Young LJ, Hearn EF, Matzuk MM, Insel TR, Winslow JT. Social amnesia in mice lacking the oxytocin gene. Nat Genet. 2000;25:284–8. https://doi.org/10.1038/77040
Augustine RA, Seymour AJ, Campbell RE, Grattan DR, Brown CH. Integrative neuro-humoral regulation of oxytocin neuron activity in pregnancy and lactation. J Neuroendocrinol. 2018. https://doi.org/10.1111/jne.12569
Grinevich V, Neumann ID. Brain oxytocin: how puzzle stones from animal studies translate into psychiatry. Mol Psychiatry. 2021;26:265–79. https://doi.org/10.1038/s41380-020-0802-9
Wang X, Zhan Y. Regulation of social recognition memory in the hippocampal circuits. Front Neural Circuits. 2022;16:839931. https://doi.org/10.3389/fncir.2022.839931
Gur R, Tendler A, Wagner S. Long-term social recognition memory is mediated by oxytocin-dependent synaptic plasticity in the medial amygdala. Biol Psychiatry. 2014;76:377–86. https://doi.org/10.1016/j.biopsych.2014.03.022
Ferguson JN, Aldag JM, Insel TR, Young LJ. Oxytocin in the medial amygdala is essential for social recognition in the mouse. J Neurosci. 2001;21:8278–85.
Tanimizu T, Kenney JW, Okano E, Kadoma K, Frankland PW, Kida S. Functional connectivity of multiple brain regions required for the consolidation of social recognition memory. J Neurosci. 2017;37:4103–16. https://doi.org/10.1523/JNEUROSCI.3451-16.2017
Cumbers MR, Chung ST, Wakerley JB. A neuromodulatory role for oxytocin within the supramammillary nucleus. Neuropeptides. 2007;41:217–26. https://doi.org/10.1016/j.npep.2007.04.004
Gould BR, Zingg HH. Mapping oxytocin receptor gene expression in the mouse brain and mammary gland using an oxytocin receptor-LacZ reporter mouse. Neuroscience. 2003;122:155–67. https://doi.org/10.1016/s0306-4522(03)00283-5
Kremarik P, Freund-Mercier MJ, Stoeckel ME. Oxytocin and vasopressin binding sites in the hypothalamus of the rat: histoautoradiographic detection. Brain Res Bull. 1995;36:195–203. https://doi.org/10.1016/0361-9230(94)00196-8
Yoshimura R, Kiyama H, Kimura T, Araki T, Maeno H, Tanizawa O, et al. Localization of oxytocin receptor messenger ribonucleic acid in the rat brain. Endocrinology. 1993;133:1239–46. https://doi.org/10.1210/endo.133.3.8396014
Swanson LW. The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res Bull. 1982;9:321–53. https://doi.org/10.1016/0361-9230(82)90145-9
Kirk IJ. Frequency modulation of hippocampal theta by the supramammillary nucleus, and other hypothalamo-hippocampal interactions: mechanisms and functional implications. Neurosci Biobehav Rev. 1998;22:291–302. https://doi.org/10.1016/s0149-7634(97)00015-8
Pan WX, McNaughton N. The supramammillary area: its organization, functions and relationship to the hippocampus. Prog Neurobiol. 2004;74:127–66. https://doi.org/10.1016/j.pneurobio.2004.09.003
Pedersen NP, Ferrari L, Venner A, Wang JL, Abbott SBG, Vujovic N, et al. Supramammillary glutamate neurons are a key node of the arousal system. Nat Commun. 2017;8:1405. https://doi.org/10.1038/s41467-017-01004-6
Renouard L, Billwiller F, Ogawa K, Clement O, Camargo N, Abdelkarim M, et al. The supramammillary nucleus and the claustrum activate the cortex during REM sleep. Sci Adv. 2015;1:e1400177. https://doi.org/10.1126/sciadv.1400177
Ikemoto S. The supramammillary nucleus mediates primary reinforcement via GABA(A) receptors. Neuropsychopharmacology. 2005;30:1088–95. https://doi.org/10.1038/sj.npp.1300660
Ikemoto S, Witkin BM, Zangen A, Wise RA. Rewarding effects of AMPA administration into the supramammillary or posterior hypothalamic nuclei but not the ventral tegmental area. J Neurosci. 2004;24:5758–65. https://doi.org/10.1523/JNEUROSCI.5367-04.2004
Ikemoto S, Qin M, Liu ZH. Primary reinforcing effects of nicotine are triggered from multiple regions both inside and outside the ventral tegmental area. J Neurosci. 2006;26:723–30. https://doi.org/10.1523/JNEUROSCI.4542-05.2006
Shin R, Ikemoto S. Administration of the GABAA receptor antagonist picrotoxin into rat supramammillary nucleus induces c-Fos in reward-related brain structures. Supramammillary picrotoxin and c-Fos expression. BMC Neurosci. 2010;11:101. https://doi.org/10.1186/1471-2202-11-101
Kesner, Shin AJ, Calva R, Don CB, Junn RF, Potter S, et al. Supramammillary neurons projecting to the septum regulate dopamine and motivation for environmental interaction in mice. Nat Commun. 2021;12:2811. https://doi.org/10.1038/s41467-021-23040-z
Chen, He S, Huang L, Boehringer AJY, Robert R, Wintzer V, et al. A hypothalamic novelty signal modulates hippocampal memory. Nature. 2020;586:270–4. https://doi.org/10.1038/s41586-020-2771-1
Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–91. https://doi.org/10.3758/bf03193146
Eliava M, Melchior M, Knobloch-Bollmann HS, Wahis J, da Silva Gouveia M, Tang Y, et al. A new population of parvocellular oxytocin neurons controlling magnocellular neuron activity and inflammatory pain processing. Neuron. 2016;89:1291–304. https://doi.org/10.1016/j.neuron.2016.01.041
Knobloch HS, Charlet A, Hoffmann LC, Eliava M, Khrulev S, Cetin AH, et al. Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron. 2012;73:553–66. https://doi.org/10.1016/j.neuron.2011.11.030
Campbell-Smith EJ, Holmes NM, Lingawi NW, Panayi MC, Westbrook RF. Oxytocin signaling in basolateral and central amygdala nuclei differentially regulates the acquisition, expression, and extinction of context-conditioned fear in rats. Learn Mem. 2015;22:247–57. https://doi.org/10.1101/lm.036962.114
Brill-Maoz N, Maroun M. Extinction of fear is facilitated by social presence: synergism with prefrontal oxytocin. Psychoneuroendocrinology. 2016;66:75–81. https://doi.org/10.1016/j.psyneuen.2016.01.003
Harony-Nicolas, H, Kay, M, du Hoffmann, J, Klein, ME, Bozdagi-Gunal, O, et al. Oxytocin improves behavioral and electrophysiological deficits in a novel Shank3-deficient rat. Elife. 2017;6. https://doi.org/10.7554/eLife.18904
Shahar-Gold H, Gur R, Wagner S. Rapid and reversible impairments of short- and long-term social recognition memory are caused by acute isolation of adult rats via distinct mechanisms. PLoS ONE. 2013;8:e65085. https://doi.org/10.1371/journal.pone.0065085
Engelmann M, Wotjak CT, Landgraf R. Social discrimination procedure: an alternative method to investigate juvenile recognition abilities in rats. Physiol Behav. 1995;58:315–21.
Mathiasen, JR, and DiCamillo, A. Novel object recognition in the rat: a facile assay for cognitive function. Curr Protoc Pharmacol. 2010;Chapter 5:Unit 5.59. https://doi.org/10.1002/0471141755.ph0559s49
Netser S, Haskal S, Magalnik H, Wagner S. A novel system for tracking social preference dynamics in mice reveals sex- and strain-specific characteristics. Mol Autism. 2017;8:53. https://doi.org/10.1186/s13229-017-0169-1
Netser, S, Haskal, S, Magalnik, H, Bizer, A, and Wagner, S. A System for tracking the dynamics of social preference behavior in small rodents. J Vis Exp. 2019. https://doi.org/10.3791/60336
McKay EC, Beck JS, Khoo SK, Dykema KJ, Cottingham SL, Winn ME, et al. Peri-Infarct Upregulation of the Oxytocin Receptor in Vascular Dementia. J Neuropathol Exp Neurol. 2019;78:436–52. https://doi.org/10.1093/jnen/nlz023
Root DH, Zhang S, Barker DJ, Miranda-Barrientos J, Liu B, Wang HL, et al. Selective Brain Distribution and Distinctive Synaptic Architecture of Dual Glutamatergic-GABAergic Neurons. Cell Rep. 2018;23:3465–79. https://doi.org/10.1016/j.celrep.2018.05.063
Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9:676–82. https://doi.org/10.1038/nmeth.2019
Netser S, Meyer A, Magalnik H, Zylbertal A, de la Zerda SH, Briller M, et al. Distinct dynamics of social motivation drive differential social behavior in laboratory rat and mouse strains. Nat Commun. 2020;11:5908 https://doi.org/10.1038/s41467-020-19569-0
Gonzalo-Ruiz A, Alonso A, Sanz JM, Llinas RR. A dopaminergic projection to the rat mammillary nuclei demonstrated by retrograde transport of wheat germ agglutinin-horseradish peroxidase and tyrosine hydroxylase immunohistochemistry. J Comp Neurol. 1992;321:300–11. https://doi.org/10.1002/cne.903210209
Borhegyi Z, Leranth C. Distinct substance P- and calretinin-containing projections from the supramammillary area to the hippocampus in rats; a species difference between rats and monkeys. Exp Brain Res. 1997;115:369–74. https://doi.org/10.1007/pl00005706
Burgunder JM, Young WS 3rd. Neurons containing cholecystokinin mRNA in the mammillary region: ontogeny and adult distribution in the rat. Cell Mol Neurobiol. 1989;9:281–94. https://doi.org/10.1007/BF00713035
Robert, V., Therreau, L., Chevaleyre, V., Lepicard, E., Viollet, C., Cognet, J., et. al. Local circuit allowing hypothalamic control of hippocampal area CA2 activity and consequences for CA1. Elife. 2021;10. https://doi.org/10.7554/eLife.63352.
Gheusi G, Bluthe RM, Goodall G, Dantzer R. Social and individual recognition in rodents: Methodological aspects and neurobiological bases. Behav Process. 1994;33:59–87. https://doi.org/10.1016/0376-6357(94)90060-4
Raam T, McAvoy KM, Besnard A, Veenema AH, Sahay A. Hippocampal oxytocin receptors are necessary for discrimination of social stimuli. Nat Commun. 2017;8:2001 https://doi.org/10.1038/s41467-017-02173-0
Phillips, ML, Robinson, HA, and Pozzo-Miller, L. Ventral hippocampal projections to the medial prefrontal cortex regulate social memory. Elife. 2019;8. https://doi.org/10.7554/eLife.44182
Wu X, Morishita W, Beier KT, Heifets BD, Malenka RC. 5-HT modulation of a medial septal circuit tunes social memory stability. Nature. 2021;599:96–101. https://doi.org/10.1038/s41586-021-03956-8
Marder E. Neuromodulation of neuronal circuits: back to the future. Neuron. 2012;76:1–11. https://doi.org/10.1016/j.neuron.2012.09.010
Stoop R. Neuromodulation by oxytocin and vasopressin. Neuron. 2012;76:142–59. https://doi.org/10.1016/j.neuron.2012.09.025
Stoop R. Neuromodulation by oxytocin and vasopressin in the central nervous system as a basis for their rapid behavioral effects. Curr Opin Neurobiol. 2014;29:187–93. https://doi.org/10.1016/j.conb.2014.09.012
Lukas M, Toth I, Veenema AH, Neumann ID. Oxytocin mediates rodent social memory within the lateral septum and the medial amygdala depending on the relevance of the social stimulus: male juvenile versus female adult conspecifics. Psychoneuroendocrinology. 2013;38:916–26. https://doi.org/10.1016/j.psyneuen.2012.09.018
Resendez SL, Namboodiri VMK, Otis JM, Eckman LEH, Rodriguez-Romaguera J, Ung RL, et al. Social stimuli induce activation of oxytocin neurons within the paraventricular nucleus of the hypothalamus to promote social behavior in male mice. J Neurosci. 2020;40:2282–95. https://doi.org/10.1523/JNEUROSCI.1515-18.2020
Horie K, Inoue K, Suzuki S, Adachi S, Yada S, Hirayama T, et al. Oxytocin receptor knockout prairie voles generated by CRISPR/Cas9 editing show reduced preference for social novelty and exaggerated repetitive behaviors. Horm Behav. 2019;111:60–69. https://doi.org/10.1016/j.yhbeh.2018.10.011
Tang Y, Benusiglio D, Lefevre A, Hilfiger L, Althammer F, Bludau A, et al. Social touch promotes interfemale communication via activation of parvocellular oxytocin neurons. Nat Neurosci. 2020;23:1125–37. https://doi.org/10.1038/s41593-020-0674-y
Penagarikano O, Lazaro MT, Lu XH, Gordon A, Dong H, Lam HA, et al. Exogenous and evoked oxytocin restores social behavior in the Cntnap2 mouse model of autism. Sci Transl Med. 2015;7:271ra278. https://doi.org/10.1126/scitranslmed.3010257
Dombret C, Nguyen T, Schakman O, Michaud JL, Hardin-Pouzet H, Bertrand MJ, et al. Loss of Maged1 results in obesity, deficits of social interactions, impaired sexual behavior and severe alteration of mature oxytocin production in the hypothalamus. Hum Mol Genet. 2012;21:4703–17. https://doi.org/10.1093/hmg/dds310
Farrell JS, Lovett-Barron M, Klein PM, Sparks FT, Gschwind T, Ortiz AL, et al. Supramammillary regulation of locomotion and hippocampal activity. Science. 2021;374:1492–6. https://doi.org/10.1126/science.abh4272
Vertes RP. PHA-L analysis of projections from the supramammillary nucleus in the rat. J Comp Neurol. 1992;326:595–622. https://doi.org/10.1002/cne.903260408
Haglund L, Swanson LW, Kohler C. The projection of the supramammillary nucleus to the hippocampal formation: an immunohistochemical and anterograde transport study with the lectin PHA-L in the rat. J Comp Neurol. 1984;229:171–85. https://doi.org/10.1002/cne.902290204
Acknowledgements
This work was supported by the Seaver Foundation for Autism Research and Treatment (HHN, KTR, MB), the National Institute of Mental Health (R01MH116108, HHN), a trainee pilot grant from the Mindich Child Health and Development Institute (KTR), Young Investigator Award from the Brain and Behavior Research Foundation (KTR) and the National Institute of Mental Health Diversity Supplement Award (MH116108-03S1, HHN, NC). AL is a recipient of Marie Curie-Skladowska fellowship. VG was supported by the German Research Foundation (DFG) grants GR 3619/15-1, GR 3619/16-1, SFB Consortium 1158-2 (VG), and GR 3619/13-1 and 3619/19-1 (SW and VG). SW was supported by ISF-NSFC joint research program (grant No. 3459/20), the Israel Science Foundation (ISF grant 1361/17), the Ministry of Science, Technology and Space of Israel (Grant No. 3-12068) and the United States-Israel Binational Science Foundation (BSF grant No. 2019186, SW and HHN). The authors would like to acknowledge Ms. Amanda Leithead and Ms. Michelle Kim’s support in reviewing the manuscript and thank the Microscopy and Advanced Bioimaging CoRE core at the Icahn School of Medicine for their guidance on imaging. Parts of Figs. 1 and 6 were created with https://BioRender.com.
Author information
Authors and Affiliations
Contributions
KTR and HHN conceptualized and designed the study. KTR performed all DREADDS and OXTR antagonist experiments, brain surgeries, viral injections, RNAscope, and acquired all imaging data. MB performed brain surgeries and viral injection for the OXT projection experiments. KN helped on the behavioral analysis. NC helped with the quantification of immunohistochemical data. AF and VG cloned and packaged the OXT-specific viruses. SN and SW provided support on the open-source behavioral analysis software. KTR and HHN, interpreted the data, prepared figures and wrote the manuscript. HHN, SW, SN and VG helped interpret the data and revise the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors report no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Thirtamara Rajamani, K., Barbier, M., Lefevre, A. et al. Oxytocin activity in the paraventricular and supramammillary nuclei of the hypothalamus is essential for social recognition memory in rats. Mol Psychiatry (2023). https://doi.org/10.1038/s41380-023-02336-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41380-023-02336-0