Abstract
Autism spectrum disorder (ASD) is a group of neurodevelopmental disorders mainly characterized by deficient sociability and repetitive behaviors. Effective treatment for the core symptoms of ASD is still lacking. Behavioral interventions show limited effectiveness, while pharmacotherapy focuses on the amelioration of secondary symptomatology. Oxytocin (OXT) is a neuropeptide known for its prosocial impact, making it a candidate drug for ASD treatment. Its alleviating effect has been and still is widely researched, but outcomes reported by clinical studies are ambiguous. We examined the effect of daily intranasal OXT (0.8 IU/kg) administration for 4 weeks on the ASD-like phenotype in Shank3−/− adult mice. Animals treated with OXT spent twice as much time interacting with the social partner as early as after 2 weeks of treatment. Furthermore, OXT-treated mice exhibited reduced explorative behavior by 50%, after 4 weeks of treatment, and a 30% reduction in repetitive behavior, 4 weeks after treatment termination. One-fold higher sociability and 30% reduced exploration due to OXT lasted up to 4 weeks following the treatment termination. However, social disinterest was elevated by roughly 10% as well, indicating a form of social ambivalence. Obtained results support the therapeutic potential of intranasally administered OXT in alleviating social shortfalls in a genetic model of ASD. Subsequent research is necessary to elucidate the benefits and risks of the long-term OXT administration, as well as its applicability in other ASD models and the potential treatment effect on social communication, which was not measured in the present study.
Similar content being viewed by others
Introduction
Autism spectrum disorder (ASD) is a group of neurodevelopmental disorders characterized by deficits in social interaction, communication, and repetitive behavior [1]. Since the etiopathogenesis is unknown, the treatment is symptomatic and inefficient. Only a limited number of ASD symptoms are treatable to this day. So far, the most prevalent treatment has been antipsychotic medication to manage irritability and hyperactivity [2]. Intense research is currently being done on novel treatment approaches targeting the core symptoms [3].
Oxytocin (OXT) is a neuropeptide best known for its crucial role in social bonding, social behavior, social recognition, and social care [4]. Previous research showed that OXT is implicated in neuroanatomical regions and neurochemical mechanisms responsible for processing social stimuli [5, 6] and its intranasal administration alters activation across brain regions of patients with ASD [7]. Furthermore, some studies have found that children suffering from ASD have lower concentrations of circulating OXT [8] and they were shown to be associated with annotated ASD risk genes [9]. Finally, a recent article showed that disruptions in OXT-related genes are associated with ASD etiology and may pose as potential biomarkers for predicting the efficacy of the OXT treatment [10]. These findings suggest its use as a possible therapeutic agent for several psychiatric disorders defined by deficits in sociability, such as ASD.
Several clinical studies have found that intranasal OXT administration improved social functioning and decreased repetitive behavior in patients suffering from ASD [11,12,13,14]. On the other hand, other studies failed to provide evidence supporting the therapeutic potential of intranasal OXT [15, 16], and a substantial number of clinical studies are still underway. However, OXT is still not being utilized due to its limited effectiveness, which appears to depend on the dosage, route of administration, and, most importantly, the characteristics of the individual patients undergoing treatment; this accents the need for further investigation of OXT as a possible therapeutic agent.
With the unclear etiopathogenesis and high heritability of ASD, genetic factors stand out as the main component involved. Thus, the genetic models of ASD pose an ideal choice for research into the disorder. One of the most prominent genetic animal models used in ASD research is the SH3 and multiple ankyrin repeat domains 3 (Shank3) gene deficiency model, which exhibits a variety of ASD-like phenotypes, such as extensive grooming indicating repetitive behavior, or abnormal social behavior [17]. Mouse models carrying Shank3 deficiency are widely preferred rodent models when it comes to ASD research. However, the research on the behavioral effects of OXT in the Shank3 animal model so far has been almost exclusively limited to rats.
An attenuating role of an acute dose of OXT on ASD phenotype was shown in Shank3-deficient rats [18] and another paper reported normative concentrations of endogenous OXT in rats with a complete deletion of Shank3 [19]. Morphological and structural neuronal deficits observed in mice carrying Shank3 deficiency have been rescued with early-life acute subcutaneous OXT administration in a recent study [20]. Acute and chronic intranasal OXT administration was also reported to produce different behavioral effects [21]. Thus, considering a more universal therapeutic response is produced following chronic administration, it seems to be a preferred choice for the OXT treatment.
To elucidate the behavioral effects of chronic non-invasive OXT treatment on ASD symptomatology, we employed the Shank3-deficient mouse model of ASD. We expected daily treatment to alleviate social deficits and manage stereotypical behavior previously reported in Shank3-deficient mice. We anticipated such an effect to last even after the treatment termination, producing a long-term behavioral response.
Material and methods
Animals
Heterozygous breeding pairs of Shank3B mice with pure C57BL/6 background were obtained from The Jackson Laboratory (JAX Stock No. #017688). Shank3B wild-type (WT) and knock-out (KO) mutant mice were generated by crossing adult (3-month-old) heterozygous males with age-matched heterozygous females Genotyping was conducted to determine the genotype of offspring. WT (female=15, male=11) and Shank3B−/− KO (female=12, male=16) adult (3-month-old) mice of both sexes with C57BL/6 background were used. Animals were group-housed (4-6 per cage) with their littermates and kept in a controlled environment of 24 ± 2 °C and 55 ± 10% humidity with ad libitum access to food and water on a 12-h light/dark cycle. The experiment was performed in accordance with the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guideline [22]. Power analysis was conducted using G*Power. No animals were excluded from the study. Animals were randomized into treatment groups, but no blinding was conducted. The experiment has been conducted in accordance with the Slovak national laws and approved by the ethics committee of the Institute of Molecular Biomedicine.
Genotyping
To confirm the genotype, end-point PCR was utilized. Tail samples (~0.2 cm) were collected from the animals at the time of weaning. Tail samples were then processed, and genomic DNA was isolated using the MyTaqTM Extract-PCR Kit (Bioline) according to the kit’s instructions Following the extraction, 2 μl of the extract containing the DNA was amplified using standard Endpoint PCR and a combination of three primers designed to identify both the WT and KO alleles (P1-Common: GAG ACT GAT CAG CGC AGT TG; P2-WT: TGA CAT AAT CGC TGG CAA AG; P3-KO: GCT ATA CGA AGT TAT GTC GAC TAG G). Combinations of primers produced a 374-bp band for the WT allele, a 153-bp band for the KO allele, and both bands for the heterozygous allele. The PCR product was run on a 1.5% agarose gel stained with the GoodViewTM Nucleic Acid Stain (Beijing SBS Genetech Co., Ltd.).
Substance administration
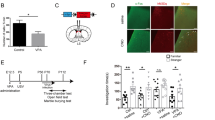
Animals were intranasally administered 0.8 IU/kg OXT (Ferring Pharmaceuticals, Switzerland) with the concentration of 1 IU/ml or saline vehicle treatment once daily between 12:00 and 15:00 according to the previously used protocol [23] for the period of 30 consecutive days. For the intranasal administration, a single channel 0.5 – 10 μl Pipette was used (Eppendorf, Germany). Drops of solution were gently placed equally on both nostrils of the mouse, which were inhaled. Administration and handling were consistent across groups and days of administration. No anesthesia was required, as the administration is non-invasive.
Behavioral testing
Behavioral testing was conducted 12 h after the last daily substance administration throughout 2 consecutive days. Mice were tested in the PhenoTyper 4500 cage (Noldus Information Technology, Wageningen, The Netherlands) including an open field arena (45 cm × 45 cm × 45 cm) for 2 behavioral assays, the Reciprocal interaction test, and the Open field test. Each test was carried out in a dimly lit room, with a room temperature of 24 ± 1 °C. All animals were habituated to the room at least 30 min prior to each test. Mouse handling and experiments were carried out by the same experimenters throughout the study. After the behavioral testing, all animals were weighed.
In the Reciprocal interaction test, subject animals were socially isolated for 24 h prior to the testing and then randomly paired with a socially novel WT animal of the same sex used as a social partner. Both animals were placed in a cage filled with sawdust bedding and left to freely interact for 10 min while being recorded. The recording was manually scored by two observers blind to the experimental groups for cumulative time spent nose-to-nose, nose-anogenital, and side-sniffing as a measure of social interaction, and self-grooming, digging, lying flat, freezing in contact, or avoiding the social partner as a measure of social disinterest. The average values of both observations were calculated and considered representative. The inter-observer variability was 4.4%.
In the Open field test, animals were individually placed into the cage and left to freely explore for 10 min while recording. Recordings were manually scored by two observers blind to the experimental groups for a cumulative time of repetitive self-injurious grooming and rearing behavior as a measure of repetitive and explorative behavior, respectively. The average values of both observations were calculated and considered representative. The inter-observer variability was 3.9%. The distance the animals traveled during the test was quantified as a measure of locomotor activity and the time they spent in the central zone was quantified as a measure of anti-anxiety behavior.
Statistical analysis
Statistical analyses were conducted using IBM SPSS Statistics 23.0 (IBM, Armonk, NY, USA). One-way ANOVA was used to compare the groups, followed by the post hoc analysis using Bonferroni correction. To elucidate the interactive effect of treatment and genotype, Two-way ANOVA was employed and to attest to the effect of time, Repeated Measures ANOVA was utilized with subsequent Bonferroni correction. Data are presented as mean ± SEM. P-values of less than 0.05 were considered significant. As no sex differences were observed, the animals were sex-pooled for statistical analysis.
Results
Sociability
Social interaction differed between the groups of animals after 2 weeks of daily OXT administration [F(2, 53) = 6.55, p = 0.003]. Saline-treated Shank3B−/− mice exhibited approximately half the time (58.9 s ± 6.4) interacting with the social-partner mouse compared to WT controls (97.5 s ± 8, p = 0.006, Fig. 1A) indicating the effect of genotype. A noteworthy effect of treatment was observed as well. Shank3B−/− mice that received OXT, compared to their saline-treated Shank3B−/− controls, exhibited double the time socially interacting with a partner (108.7 s ± 8.9, p < 0.001, Fig. 1A). Similar results were observed in social interaction after 4 weeks of OXT treatment between the groups [F(2, 53) = 9.55, p < 0.001]. Shank3B−/− mice treated with saline consistently showed deficits in sociability (56.7 s ± 12.3) with roughly 50% less time spent interacting with a social-partner animal than the WT control mice did (98.7 s ± 7, p = 0.003, Fig. 1A). Furthermore, Shank3B−/− animals treated with OXT interacted with the social partner twice as much compared to the saline-treated Shank3B−/− mice (117.8 s ± 8.4, p < 0.001, Fig. 1A). Finally, when the animals were behaviorally tested 4 weeks following the OXT treatment termination, distinct differences between the groups were observed in social interaction also [F(2, 53) = 6.08, p = 0.004]. Saline-treated Shank3B−/− controls displayed half the time interacting with a socially novel mouse (43.2 s ± 7.3) compared to what was detected in WT controls (96.5 s ± 9.3, p = 0.001, Fig. 1A). Most importantly, OXT-treated Shank3B−/− mice exhibited over twice as much time spent in social interaction than saline-treated Shank3B−/− mice (101.1 s ± 13.6, p = 0.001, Fig. 1A). No effect of time was observed in social interaction.
The results obtained when quantifying social disinterest indicate mostly complimentary outcomes to the ones observed in social interaction. During the measurement after 2 weeks of daily administration, animal groups differed significantly in disregard towards the social partner mouse in the Reciprocal interaction test [F(2, 53) = 15.66, p < 0.001]. Shank3B−/− mice treated with saline spend almost three times more time avoiding contact with a partner mouse (149.8 s ± 16) than the WT mice (60.8 s ± 6.1, p < 0.001, Fig. 1B). On the other hand, OXT-treated Shank3B−/− mice exhibited roughly 50% less time spent avoiding the social partner compared to controls (89.6 s ± 13.2, p = 0.008, Fig. 1B). When looking at the results from the measurement after 4 weeks of daily OXT administration, once again a significant difference between groups was recorded [F(2, 53) = 6.52, p = 0.003]. Saline-treated Shank3B−/− mice spent 25% more time not interested in their social-partner mouse (116.1 s ± 17.3) than the WTs (56 s ± 6.9, p = 0.006), while Shank3B−/− mice treated with OXT did not significantly differ from their Shank3B−/− controls (88.6 s ± 14.3, p = 0.234, Fig. 1B). Upon investigating the social disinterest 4 weeks following the treatment termination, groups differed considerably [F(2, 53) = 18.96, p < 0.001]. A large part of this difference was made up of saline-treated Shank3B−/− mice, which spend more than three times longer disregarding the social-partner mouse (95.2 s ± 19.9) compared to the WT mice (29.4 s ± 5.9, p < 0.001). No significant differences were shown between Shank3B−/− mice treated with OXT and Shank3B−/− mice treated with saline. Finally, the interactive effect of grouping and time was observed [F(2, 51) = 8.89, p < 0.001] across the animal groups. Both WT mice and Shank3B−/− mice treated with saline exhibited a consistent decrease in social disinterest in time, however, this was not true for the OXT-treated Shank3B−/− mice which exhibited an increasing tendency over the testing time points [F(2, 32) = 6.76, p = 0.019].
Repetitive behavior
The repetitive behavior differed between the groups after 2 weeks of treatment [F(2, 51) = 6.90, p = 0.002]. A main effect of genotype was observed, as the saline-treated Shank3B−/− mice exhibited over twice the amount of self-injurious grooming (57.8 s ± 12.9) compared to the WT controls treated with saline (23.6 s ± 2.4, p = 0.002, Fig. 2A). However, Shank3B−/− mice treated with OXT did not differ when compared to their Shank3B−/− controls treated with saline (39.9 s ± 6.9, p = 0.194). Data analysis at 4 weeks since the treatment started revealed differences between groups in repetitive behavior as well [F(2, 51) = 12.99, p < 0.001]. Shank3B−/− mice treated with saline spend 70% more time grooming (74.3 s ± 14.6) than the WT controls (21.8 s ± 1.9, p = 0.005, Fig. 2A). Once again, OXT-treated Shank3B−/− mice did not exhibit reduced self-grooming compared to the saline-treated Shank3B−/− control mice (50.1 s ± 8.5, p = 0.137). Finally, 4 weeks following the treatment termination, the difference between the groups was recorded [F(2, 51) = 36.56, p < 0.001]. Similar to the previous time points, saline-treated Shank3B−/− mice exhibited 5-times more self-grooming (84.2 s ± 12.7) than the WT controls (13.4 s ± 2.3, p < 0.001, Fig. 2A). Interestingly, this time the OXT-treated Shank3B−/− mice exhibited reduced self-grooming by a third compared to the saline-treated Shank3B−/− mice (56.3 s ± 5.9, p = 0.036, Fig. 2A). However, no effect of time was implicated in this finding [F(2, 32) = 2.38, p = 0.108].
Explorative behavior
Two weeks after OXT administration, groups did not differ in explorative behavior [F(2, 32) = 0.49, p = 0.616]. However, after 4 weeks of treatment, a difference was observed between the groups [F(2, 32) = 14.3, p < 0.001]. Saline-treated Shank3B−/− mice spend about 20% more time exploring their surroundings than the WT controls (113.1 s ± 7.1, p = 0.021, Fig. 2B). Furthermore, OXT-treated Shank3B−/− mice exhibited almost half as much time exploring compared to the saline-treated Shank3B−/− mice (81.4 s ± 4.62, p < 0.001). Similarly, explorative behavior differed between the groups even 4 weeks after the treatment termination [F(2, 32) = 54.4, p < 0.001]. This time, saline-treated Shank3B−/− mice exhibited about 20% lower explorative behavior (113.7 s ± 8.1) compared to the WT controls (147.3 s ± 3.3, p = 0.002, Fig. 2B). Most importantly, Shank3B−/− mice treated with OXT exhibited 30% less interest in exploring their surroundings when compared to the saline-treated Shank3B−/− control mice (76.9 s ± 5.9, p < 0.001, Fig. 2B).
Anxiety-like behavior
No differences between the groups were observed in anxiety-like behavior either after 2 weeks of treatment [F(2, 51) = 0.46, p = 0.632], or after 4 weeks of treatment [F(2, 51) = 3.21, p = 0.481], or 4 weeks following the treatment termination [F(2, 51) = 3.57, p = 0.352, Fig. 3A].
Locomotor activity and body weight
The animals did not differ in locomotor activity after 2 weeks [F(2, 51) = 1.68, p = 0.195], and 4 weeks of treatment [F(2, 51) = 3.89, p = 0.271], or 4 weeks after the treatment was suspended [F(2, 51) = 1.34, p = 0.269, Fig. 3B]. Finally, the groups did not differ in body weight at any time points: after 2 weeks [F(2, 51) = 2.94, p = 0.062], or 4 weeks of the treatment [F(2, 51) = 3.19, p = 0.49], and 4 weeks after the administration was terminated [F(2, 51) = 2.92, p = 0.063, Fig. 3C].
Discussion
This study was, to our knowledge, the first to examine the effect of chronic intranasal OXT treatment on autistic phenotype in the Shank3B−/− mutant mouse model of ASD. Daily intranasal administration of OXT prevented social deficits exhibited by Shank3B−/− mice as early as after 2 weeks of treatment, but further manifested in a form of social ambivalence. Treated animals exhibited a reduced interest in their surroundings and slightly attenuated repetitive self-injurious behavior 4 weeks after the treatment was terminated.
Amelioration of social behavior due to OXT in the current study is a finding consistent across several previous studies with BALB/cByJ and C58/J mice models of ASD [24, 25] despite their variations in volumes, lengths, and routes of administration, which might produce different prosocial effects [21]. However, in the BTBR mouse model [23], with which our study shared a substantial portion of methodology, such as administration protocol, dose, and behavioral assays, no effect of OXT treatment on sociability was recorded. The differences in results could be partly explained by the disparity of age in which the animals were tested in both studies; our study was done on adult mice, whereas Bales et al. [23] used mice in adolescence. Furthermore, the authors claim to observe no effects of long-term exposure to OXT on social interaction but assessed the reciprocal social interaction only 3 days following the daily OXT administration, starting at weaning. It is thus plausible that the substance in the presented dose did not take a sufficient effect at the time of assessment. A recent study investigating the effects of OXT administered by intracranial injections in Shank3−/− rats has noted an impairing effect on social memory, but not on social interaction [18]. Presumably, the differences in the administration route as well as in the genetic background of subjects might be causing disparities in the results. Finally, a recent study recorded no effect of intranasal OXT treatment on treating the sociability deficit across three different mouse models of autism, including the Shank3B−/− mutant mice [26]. However, it is important to pinpoint that the Three-chamber social interaction test was utilized to quantify sociability in the article. Several previous studies reported the Three-chamber social interaction test not being able to intercept social deficits, whereas the Reciprocal interaction test was [27, 28]. Thus, the validity of the assay could be in question and can play a role in the disparity of recorded results in sociability.
Furthermore, we observed a steady increase in social disinterest in OXT-treated animals across the testing time points. This finding suggests a form of socially ambivalent behavior, where on one hand, daily administration of OXT ameliorated social deficits by making the animals more interested in a social partner, but on the other hand, it also steadily increased social irritability following contact with the social partner as well. Such duality was not yet reported in similar research, so we can only hypothesize about the mechanisms at play. There is evidence pointing to changes in OXT receptor expression and regulation following the stimulation by the exogenous neuropeptide [23]. In line with this, it has been reported earlier that the stimulation of OXT receptors increases the expression of neurexins and scaffolding proteins, such as SHANK3 in a cell-specific manner [29], rescuing the deficits caused by the mutation to some degree. However, pharmacologically increased OXT availability over a longer period is known to lead to receptor downregulation, which can be manifested in paradoxical effects [30,31,32]. Thus, presumably, it can be the cause of the observed socially ambivalent behavior. However, as we did not record the expression of the OXT receptors in the brain, further research is required to elucidate this hypothesis.
Repetitive behavior was consistently impaired in Shank3B−/− mice in our study and the OXT administration only partially ameliorated the deficit. The attenuation of extensive repetitions in animals treated with OXT was observed only 4 weeks after the daily treatment was terminated. Interestingly though, such an outcome could shed some new light on inconclusive findings provided in literature so far. It was previously reported that acute OXT treatment in young adult C58/J mice decreased repetitive grooming [25], and similarly to this, in a valproic acid-induced rat model of autism, repetitive behavior was rescued by OXT administration [33]. Besides the differences in the age of subjects, mentioned studies used intraperitoneal and subcutaneous routes of administration, respectively, which are reported to provide a more systemic substance effect [34]. On the other hand, using the intranasal route of OXT administration for 7 successive days does not seem to mediate such a therapeutic effect on repetitive behavior [23, 35]. Therefore, this evidence points to a possibility that for OXT to influence repetitive behavior, a more systemic effect needs to be induced, possibly targeting relevant underlying mechanisms not yet elucidated. In addition, it could be presumed that intranasal OXT is fully able to impact excessive repetitive behavior if delivered consistently over a longer period. Taken together, the route and time of administration can arbitrate an attenuating effect of OXT on repetitive behavior.
Animals that received OXT daily in our experiment exhibited a reduced interest in exploring their surroundings following administration over a longer period. Previously reported effects of chronic OXT increasing the exploration [21] were not supported by our results. As the effect in the mentioned paper was dose-dependent, i.e., only observed in the group with an OXT dose of 5 IU/kg, which is an over 5-fold higher dose than the one used in our study, we expect the lower dose to be the key factor behind the noted discrepancies. There is some evidence reporting OXT having sedative effects in rhesus monkeys and rabbits [36], but only a trend of mild sedation was observed in research with BTBR mice receiving OXT treatment [23]. Although the OXT-treated Shank3B−/− mice in our experiment exhibited reduced explorative behavior mediated by treatment, they showed normative locomotion, anxiety-like behavior, and body weight change. This suggests the possible OXT-induced sedative effect we observed was not neophobia- or activity-driven, but rather specifically reduced interest in the surroundings of the animal. Subsequent research into this is therefore needed.
Our findings, however, should be understood with several limitations in mind. The concentrations of the absorbed OXT, as well as its kinetics, were not recorded, therefore we lack information on effect duration. Furthermore, as ASD is primarily prevalent at an early age, adulthood might not be the most optimal age for modeling the disorder. Although we set out to investigate the OXT effect on the core ASD symptomatology, we lacked any measure for the deficits in communication, which are highly prevalent in autism. On the other hand, as impaired sociability is arguably the most debilitating condition of ASD, we illustrated a substantial attenuative effect of the substance, rendering the social deficiency to none.
To what degree the reported results can be translated to human research and if at all, relies on clinical studies. Recently published results from clinical trials suggest little to no therapeutic effect of OXT in patients with autism [16, 37,38,39], but others show dose-dependent [14], and age-dependent effects [40]. Given the heterogeneity as vast as in ASD, it is highly probable the same therapeutic reagent would not apply to all patients. We demonstrated that chronic intranasal OXT exposure can reliably ameliorate social deficits in the Shank3B−/− mouse model of autism as early as after 2 weeks of administration. Furthermore, chronic treatment seems to have the potential to reduce extensive repetitive behavior as well. While the SHANK3 mutation is prevalent in only about 1% of patients with ASD [41], intranasal OXT treatment might pose an effective treatment option specifically for this subset.
Data availability
Data from this study are available from the authors upon reasonable request.
References
American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5-TR. 5th edition revised. Arlington, VA: American Psychiatric Association; 2022. p. 947.
Fieiras C, Chen MH, Escobar Liquitay CM, Meza N, Rojas V, Franco JVA, et al. Risperidone and aripiprazole for autism spectrum disorder in children: an overview of systematic reviews. BMJ Evid Based Med. 2022;7–14. https://doi.org/10.1136/bmjebm-2021-111804.
Frye RE. Social skills deficits in autism spectrum disorder: potential biological origins and progress in developing therapeutic agents. CNS Drugs. 2018;32:713–34.
Lim MM, Bielsky IF, Young LJ. Neuropeptides and the social brain: potential rodent models of autism. Int J Dev Neurosci. 2005;23:235–43.
Andari E, Nishitani S, Kaundinya G, Caceres GA, Morrier MJ, Ousley O, et al. Epigenetic modification of the oxytocin receptor gene: implications for autism symptom severity and brain functional connectivity. Neuropsychopharmacology. 2020;45:1150–8.
Benner S, Aoki Y, Watanabe T, Endo N, Abe O, Kuroda M, et al. Neurochemical evidence for differential effects of acute and repeated oxytocin administration. Mol Psychiatry. 2021;26:710–20.
Fathabadipour S, Mohammadi Z, Roshani F, Goharbakhsh N, Alizadeh H, Palizgar F, et al. The neural effects of oxytocin administration in autism spectrum disorders studied by fMRI: a systematic review. J Psychiatr Res. 2022;154:80–90.
Rutigliano G, Rocchetti M, Paloyelis Y, Gilleen J, Sardella A, Cappucciati M, et al. Peripheral oxytocin and vasopressin: biomarkers of psychiatric disorders? A comprehensive systematic review and preliminary meta-analysis. Psychiatry Res. 2016;241:207–20.
Siecinski SK, Giamberardino SN, Spanos M, Hauser AC, Gibson JR, Chandrasekhar T, et al. Genetic and epigenetic signatures associated with plasma oxytocin levels in children and adolescents with autism spectrum disorder. Autism Res. 2023;16:502–23.
Wang T, Zhao T, Liu L, Teng H, Fan T, Li Y, et al. Integrative analysis prioritised oxytocin-related biomarkers associated with the aetiology of autism spectrum disorder. EBioMedicine. 2022;81:104091.
Andari E, Duhamel JR, Zalla T, Herbrecht E, Leboyer M, Sirigu A. Promoting social behavior with oxytocin in high-functioning autism spectrum disorders. PNAS. 2010;107:4389–94.
Hollander E, Novotny S, Hanratty M, Yaffe R, DeCaria CM, Aronowitz BR, et al. Oxytocin infusion reduces repetitive behaviors in adults with autistic and Asperger’s disorders. Neuropsychopharmacology. 2003;28:193–8.
Kosaka H, Okamoto Y, Munesue T, Yamasue H, Inohara K, Fujioka T, et al. Oxytocin efficacy is modulated by dosage and oxytocin receptor genotype in young adults with high-functioning autism: a 24-week randomized clinical trial. Transl Psychiatry. 2016;6:e872–872.
Yamasue H, Kojima M, Kuwabara H, Kuroda M, Matsumoto K, Kanai C, et al. Effect of a novel nasal oxytocin spray with enhanced bioavailability on autism: a randomized trial. Brain. 2022;145:490–9.
Cacciotti-Saija C, Langdon R, Ward PB, Hickie IB, Scott EM, Naismith SL, et al. A double-blind randomized controlled trial of oxytocin nasal spray and social cognition training for young people with early psychosis. Schizophr Bull. 2015;41:483–93.
Sikich L, Kolevzon A, King BH, McDougle CJ, Sanders KB, Kim SJ, et al. Intranasal oxytocin in children and adolescents with autism spectrum disorder. N Engl J Med. 2021;385:1462–73.
Peça J, Feliciano C, Ting JT, Wang W, Wells MF, Venkatraman TN, et al. Shank3 mutant mice display autistic-like behaviours and striatal dysfunction. Nature. 2011;472:437–42.
Harony-Nicolas H, Kay M, du Hoffmann J, Klein ME, Bozdagi-Gunal O, et al. Oxytocin improves behavioral and electrophysiological deficits in a novel Shank3-deficient rat. Elife. 2017;6:e18904.
Song TJ, Lan XY, Wei MP, Zhai FJ, Boeckers TM, Wang JN, et al. Altered behaviors and impaired synaptic function in a novel rat model with a complete Shank3 deletion. Front Cell Neurosci. 2019;13:111.
Reichova A, Bacova Z, Bukatova S, Kokavcova M, Meliskova V, Frimmel K, et al. Abnormal neuronal morphology and altered synaptic proteins are restored by oxytocin in autism-related SHANK3 deficient model. Mol Cell Endocrinol. 2020;518:110924.
Huang H, Michetti C, Busnelli M, Managò F, Sannino S, Scheggia D, et al. Chronic and acute intranasal oxytocin produce divergent social effects in mice. Neuropsychopharmacology. 2014;39:1102–14.
Sert, du NP, Ahluwalia A, Alam S, Avey MT, Baker M, et al. Reporting animal research: explanation and elaboration for the ARRIVE guidelines 2.0. PLOS Biol. 2020;18:e3000411.
Bales KL, Solomon M, Jacob S, Crawley JN, Silverman JL, Larke RH, et al. Long-term exposure to intranasal oxytocin in a mouse autism model. Transl Psychiatry. 2014;4:e480.
Hara Y, Ago Y, Higuchi M, Hasebe S, Nakazawa T, Hashimoto H, Matsuda T, Takuma K. Oxytocin attenuates deficits in social interaction but not recognition memory in a prenatal valproic acid-induced mouse model of autism. Horm Behav. 2017;96:130–6.
Teng BL, Nonneman RJ, Agster KL, Nikolova VD, Davis TT, Riddick NV, et al. Prosocial effects of oxytocin in two mouse models of autism spectrum disorders. Neuropharmacology. 2013;72:187–96.
Lindenmaier Z, Ellegood J, Stuive M, Easson K, Yee Y, Fernandes D, et al. Examining the effect of chronic intranasal oxytocin administration on the neuroanatomy and behavior of three autism-related mouse models. Neuroimage. 2022;257:119243.
Jaramillo TC, Speed HE, Xuan Z, Reimers JM, Escamilla CO, Weaver TP, et al. Novel Shank3 mutant exhibits behaviors with face validity for autism and altered striatal and hippocampal function. Autism Res. 2017;10:42–65.
Yang M, Bozdagi O, Scattoni ML, Wöhr M, Roullet FI, Katz AM, et al. Reduced excitatory neurotransmission and mild autism-relevant phenotypes in adolescent Shank3 null mutant mice. J Neurosci. 2012;32:6525–41.
Zatkova M, Reichova A, Bacova Z, Bakos J. Activation of the oxytocin receptor modulates the expression of synaptic adhesion molecules in a cell-specific manner. J Mol Neurosci. 2019;68:171–80.
Conti F, Sertic S, Reversi A, Chini B. Intracellular trafficking of the human oxytocin receptor: evidence of receptor recycling via a Rab4/Rab5 “short cycle”. American J Physiol-Endocrinol Metab. 2009;296:E532–42.
Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol Rev. 2001;81:629–83.
Jurek B, Neumann ID. The oxytocin receptor: from intracellular signaling to behavior. Physiol Rev. 2018;98:1805–908.
Dai YC, Zhang HF, Schön M, Böckers TM, Han SP, Han JS, et al. Neonatal oxytocin treatment ameliorates autistic-like behaviors and oxytocin deficiency in valproic acid-induced rat model of autism. Front Cell Neurosci [Internet]. 2018 [cited 24 Feb 2022];12. Available from: https://www.frontiersin.org/article/10.3389/fncel.2018.00355.
Smith AS, Korgan AC, Young WS. Oxytocin delivered nasally or intraperitoneally reaches the brain and plasma of normal and oxytocin knockout mice. Pharmacol Res. 2019;146:104324.
Calcagnoli F, Kreutzmann JC, de Boer SF, Althaus M, Koolhaas JM. Acute and repeated intranasal oxytocin administration exerts anti-aggressive and pro-affiliative effects in male rats. Psychoneuroendocrinology. 2015;51:112–21.
Hess L, Votava M, Málek J, Kurzová A, Slíva J. Sedative effects of intranasal oxytocin in rabbits and rhesus monkeys. Physiol Res. 2016;65:S473–80.
Bernaerts S, Boets B, Bosmans G, Steyaert J, Alaerts K. Behavioral effects of multiple-dose oxytocin treatment in autism: a randomized, placebo-controlled trial with long-term follow-up. Mol Autism. 2020;11:6.
Bernaerts S, Boets B, Steyaert J, Wenderoth N, Alaerts K. Oxytocin treatment attenuates amygdala activity in autism: a treatment-mechanism study with long-term follow-up. Transl Psychiatry. 2020;10:1–12.
Kiani Z, Farkhondeh T, Aramjoo H, Aschner M, Beydokhti H, Esmaeili A, et al. Oxytocin effect in adult patients with autism: an updated systematic review and meta-analysis of randomized controlled trials. CNS Neurol Disord Drug Targets. 2023;22:906–15.
Guastella AJ, Boulton KA, Whitehouse AJO, Song YJ, Thapa R, Gregory SG, et al. The effect of oxytocin nasal spray on social interaction in young children with autism: a randomized clinical trial. Mol Psychiatry. 2023;28:834–42.
Boccuto L, Lauri M, Sarasua SM, Skinner CD, Buccella D, Dwivedi A, et al. Prevalence of SHANK3 variants in patients with different subtypes of autism spectrum disorders. Eur J Hum Genet. 2013;21:310–6.
Acknowledgements
The research was supported by Slovak Agency for Research and Development No: APVV-20-0185 and APVV-20-0070.
Funding
Open access funding provided by The Ministry of Education, Science, Research and Sport of the Slovak Republic in cooperation with Centre for Scientific and Technical Information of the Slovak Republic.
Author information
Authors and Affiliations
Contributions
JS darfted the manuscript. JS, MM and AF carried out the animal experiment, including oxytocin intervention and behavioral testing, and did the statistical analysis. ER and VB supervised the project, proposed the experiment design and revised the results including their interpretation. DO and PC conceived the study, provided scientific guidance and received the funding for the study. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Szabó, J., Mlynár, M., Feješ, A. et al. Intranasal oxytocin in a genetic animal model of autism. Mol Psychiatry (2023). https://doi.org/10.1038/s41380-023-02330-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41380-023-02330-6