Abstract
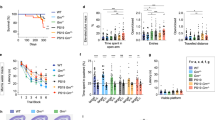
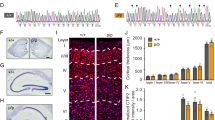
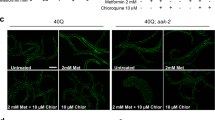
Tau protein is implicated in the pathogenesis of Alzheimer’s disease (AD) and other tauopathies, but its physiological function is in debate. Mostly explored in the brain, tau is also expressed in the pancreas. We further explored the mechanism of tau’s involvement in the regulation of glucose-stimulated insulin secretion (GSIS) in islet β-cells, and established a potential relationship between type 2 diabetes mellitus (T2DM) and AD. We demonstrate that pancreatic tau is crucial for insulin secretion regulation and glucose homeostasis. Tau levels were found to be elevated in β-islet cells of patients with T2DM, and loss of tau enhanced insulin secretion in cell lines, drosophila, and mice. Pharmacological or genetic suppression of tau in the db/db diabetic mouse model normalized glucose levels by promoting insulin secretion and was recapitulated by pharmacological inhibition of microtubule assembly. Clinical studies further showed that serum tau protein was positively correlated with blood glucose levels in healthy controls, which was lost in AD. These findings present tau as a common therapeutic target between AD and T2DM.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Biessels GJ, Staekenborg S, Brunner E, Brayne C, Scheltens P. Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol. 2006;5:64–74.
Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14:88–98.
Grodstein F, Chen J, Wilson RS, Manson JE. Type 2 diabetes and cognitive function in community-dwelling elderly women. Diabetes care. 2001;24:1060–5.
Luchsinger JA, Tang MX, Shea S, Mayeux R. Hyperinsulinemia and risk of Alzheimer disease. Neurology. 2004;63:1187–92.
Peila R, Rodriguez BL, Launer LJ. Type 2 diabetes, APOE gene, and the risk for dementia and related pathologies: The Honolulu-Asia Aging Study. Diabetes. 2002;51:1256–62.
Xu WL, Qiu CX, Wahlin A, Winblad B, Fratiglioni L. Diabetes mellitus and risk of dementia in the Kungsholmen project: a 6-year follow-up study. Neurology. 2004;63:1181–6.
Craft S, Peskind E, Schwartz MW, Schellenberg GD, Raskind M, Porte D Jr. Cerebrospinal fluid and plasma insulin levels in Alzheimer’s disease: relationship to severity of dementia and apolipoprotein E genotype. Neurology. 1998;50:164–8.
Arnold SE, Arvanitakis Z, Macauley-Rambach SL, Koenig AM, Wang HY, Ahima RS, et al. Brain insulin resistance in type 2 diabetes and Alzheimer disease: concepts and conundrums. Nat Rev Neurol. 2018;14:168–81.
Kshirsagar V, Thingore C, Juvekar A. Insulin resistance: a connecting link between Alzheimer’s disease and metabolic disorder. Metab Brain Dis. 2021;36:67–83.
Rad SK, Arya A, Karimian H, Madhavan P, Rizwan F, Koshy S, et al. Mechanism involved in insulin resistance via accumulation of β-amyloid and neurofibrillary tangles: link between type 2 diabetes and Alzheimer’s disease. Drug Des Dev Ther. 2018;12:3999–4021.
Chen W, Huang Q, Lazdon EK, Gomes A, Wong M, Stephens E, et al. Loss of insulin signaling in astrocytes exacerbates Alzheimer-like phenotypes in a 5xFAD mouse model. Proc Natl Acad Sci USA. 2023;120:e2220684120.
Miklossy J, Qing H, Radenovic A, Kis A, Vileno B, Làszló F, et al. Beta amyloid and hyperphosphorylated tau deposits in the pancreas in type 2 diabetes. Neurobiol Aging. 2010;31:1503–15.
Wijesekara N, Ahrens R, Sabale M, Wu L, Ha K, Verdile G, et al. Amyloid-β and islet amyloid pathologies link Alzheimer’s disease and type 2 diabetes in a transgenic model. FASEB J. 2017;31:5409–18.
Baas PW, Qiang L. Tau: It’s Not What You Think. Trends Cell Biol. 2019;29:452–61.
Qiang L, Sun X, Austin TO, Muralidharan H, Jean DC, Liu M, et al. Tau Does Not Stabilize Axonal Microtubules but Rather Enables Them to Have Long Labile Domains. Curr Biol. 2018;28:2181–2189.e2184.
Morris M, Maeda S, Vossel K, Mucke L. The many faces of tau. Neuron. 2011;70:410–26.
Baglietto-Vargas D, Shi J, Yaeger DM, Ager R, LaFerla FM. Diabetes and Alzheimer’s disease crosstalk. Neurosci Biobehav Rev. 2016;64:272–87.
Marciniak E, Leboucher A, Caron E, Ahmed T, Tailleux A, Dumont J, et al. Tau deletion promotes brain insulin resistance. J Exp Med. 2017;214:2257–69.
McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr, Kawas CH, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263–9.
Masellis M, Sherborn K, Neto P, Sadovnick DA, Hsiung GY, Black SE, et al. Early-onset dementias: diagnostic and etiological considerations. Alzheimers Res Ther. 2013;5:S7.
Jiang L, Ding X, Wang W, Yang X, Li T, Lei P. Head-to-Head Comparison of Different Blood Collecting Tubes for Quantification of Alzheimer’s Disease Biomarkers in Plasma. Biomolecules. 2022;12:1194.
Dawson HN, Ferreira A, Eyster MV, Ghoshal N, Binder LI, Vitek MP. Inhibition of neuronal maturation in primary hippocampal neurons from tau deficient mice. J Cell Sci. 2001;114:1179–87.
Muka T, Nano J, Jaspers L, Meun C, Bramer WM, Hofman A, et al. Associations of Steroid Sex Hormones and Sex Hormone-Binding Globulin With the Risk of Type 2 Diabetes in Women: A Population-Based Cohort Study and Meta-analysis. Diabetes. 2017;66:577–86.
De Paoli M, Zakharia A, Werstuck GH. The Role of Estrogen in Insulin Resistance: A Review of Clinical and Preclinical Data. Am J Pathol. 2021;191:1490–8.
Mangiafico SP, Lim SH, Neoh S, Massinet H, Joannides CN, Proietto J, et al. A primary defect in glucose production alone cannot induce glucose intolerance without defects in insulin secretion. J Endocrinol. 2011;210:335–47.
Zhang E, Mohammed Al-Amily I, Mohammed S, Luan C, Asplund O, Ahmed M, et al. Preserving Insulin Secretion in Diabetes by Inhibiting VDAC1 Overexpression and Surface Translocation in β Cells. Cell Metab. 2019;29:64–77.e66.
Needham BE, Wlodek ME, Ciccotosto GD, Fam BC, Masters CL, Proietto J, et al. Identification of the Alzheimer’s disease amyloid precursor protein (APP) and its homologue APLP2 as essential modulators of glucose and insulin homeostasis and growth. J Pathol. 2008;215:155–63.
Yang CH, Mangiafico SP, Waibel M, Loudovaris T, Loh K, Thomas HE, et al. E2f8 and Dlg2 genes have independent effects on impaired insulin secretion associated with hyperglycaemia. Diabetologia. 2020;63:1333–48.
Lacy PE, Kostianovsky M. Method for the isolation of intact islets of Langerhans from the rat pancreas. Diabetes. 1967;16:35–39.
Gotoh M, Maki T, Kiyoizumi T, Satomi S, Monaco AP. An improved method for isolation of mouse pancreatic islets. Transplantation. 1985;40:437–8.
Zhu X, Hu R, Brissova M, Stein RW, Powers AC, Gu G, et al. Microtubules Negatively Regulate Insulin Secretion in Pancreatic β Cells. Dev Cell. 2015;34:656–68.
Bugliani M, Liechti R, Cheon H, Suleiman M, Marselli L, Kirkpatrick C, et al. Microarray analysis of isolated human islet transcriptome in type 2 diabetes and the role of the ubiquitin-proteasome system in pancreatic beta cell dysfunction. Mol Cell Endocrinol. 2013;367:1–10.
Segerstolpe A, Palasantza A, Eliasson P, Andersson EM, Andreasson AC, Sun X, et al. Single-Cell Transcriptome Profiling of Human Pancreatic Islets in Health and Type 2 Diabetes. Cell Metab. 2016;24:593–607.
Burnouf S, Gronke S, Augustin H, Dols J, Gorsky MK, Werner J, et al. Deletion of endogenous Tau proteins is not detrimental in Drosophila. Sci Rep. 2016;6:23102.
Cleveland DW, Hwo SY, Kirschner MW. Purification of tau, a microtubule-associated protein that induces assembly of microtubules from purified tubulin. J Mol Biol. 1977;116:207–25.
Cleveland DW, Hwo SY, Kirschner MW. Physical and chemical properties of purified tau factor and the role of tau in microtubule assembly. J Mol Biol. 1977;116:227–47.
Zhu X, Hu R, Brissova M, Stein RW, Powers AC, Gu G, et al. Microtubules Negatively Regulate Insulin Secretion in Pancreatic beta Cells. Dev Cell. 2015;34:656–68.
Min SW, Chen X, Tracy TE, Li Y, Zhou Y, Wang C, et al. Critical role of acetylation in tau-mediated neurodegeneration and cognitive deficits. Nat Med. 2015;21:1154–62.
VandeVrede L, Dale ML, Fields S, Frank M, Hare E, Heuer HW, et al. Open-Label Phase 1 Futility Studies of Salsalate and Young Plasma in Progressive Supranuclear Palsy. Mov Disord Clin Pract. 2020;7:440–7.
Goldfine AB, Fonseca V, Jablonski KA, Chen YD, Tipton L, Staten MA, et al. Salicylate (salsalate) in patients with type 2 diabetes: a randomized trial. Ann Intern Med. 2013;159:1–12.
Goldfine AB, Silver R, Aldhahi W, Cai D, Tatro E, Lee J, et al. Use of salsalate to target inflammation in the treatment of insulin resistance and type 2 diabetes. Clin Transl Sci. 2008;1:36–43.
Kadohara K, Sato I, Kawakami K. Diabetes mellitus and risk of early-onset Alzheimer’s disease: a population-based case-control study. Eur J Neurol. 2017;24:944–9.
Nam E, Lee YB, Moon C, Chang KA. Serum Tau Proteins as Potential Biomarkers for the Assessment of Alzheimer’s Disease Progression. Int J Mol Sci. 2020;21:5007.
Ashcroft FM, Rorsman P. Diabetes mellitus and the β cell: the last ten years. Cell. 2012;148:1160–71.
Jung HJ, Kim YJ, Eggert S, Chung KC, Choi KS, Park SA. Age-dependent increases in tau phosphorylation in the brains of type 2 diabetic rats correlate with a reduced expression of p62. Exp Neurol. 2013;248C:441–50.
Kim B, Backus C, Oh S, Hayes JM, Feldman EL. Increased tau phosphorylation and cleavage in mouse models of type 1 and type 2 diabetes. Endocrinology. 2009;150:5294–301.
Liu F, Shi J, Tanimukai H, Gu J, Gu J, Grundke-Iqbal I, et al. Reduced O-GlcNAcylation links lower brain glucose metabolism and tau pathology in Alzheimer’s disease. Brain. 2009;132:1820–32.
Maj M, Gartner W, Ilhan A, Neziri D, Attems J, Wagner L. Expression of TAU in insulin-secreting cells and its interaction with the calcium-binding protein secretagogin. J Endocrinol. 2010;205:25–36.
Zhou R, Hu W, Dai CL, Gong CX, Iqbal K, Zhu D, et al. Expression of Microtubule Associated Protein Tau in Mouse Pancreatic Islets Is Restricted to Autonomic Nerve Fibers. J Alzheimer’s Dis. 2020;75:1339–49.
Betrie AH, Ayton S, Bush AI, Angus JA, Lei P, Wright CE. Evidence of a Cardiovascular Function for Microtubule-Associated Protein Tau. J Alzheimers Dis. 2017;56:849–60.
Maj M, Hoermann G, Rasul S, Base W, Wagner L, Attems J. The Microtubule-Associated Protein Tau and Its Relevance for Pancreatic Beta Cells. J Diabetes Res. 2016;2016:1964634.
Das UN. Colchicine in diabetes mellitus. J Assoc Physicians India. 1993;41:213.
Qiang L, Yu W, Andreadis A, Luo M, Baas PW. Tau protects microtubules in the axon from severing by katanin. J Neurosci. 2006;26:3120–9.
Kellogg EH, Hejab NMA, Poepsel S, Downing KH, DiMaio F, Nogales E. Near-atomic model of microtubule-tau interactions. Science. 2018;360:1242–6.
Tapia-Rojas C, Cabezas-Opazo F, Deaton CA, Vergara EH, Johnson GVW, Quintanilla RA. It’s all about tau. Prog Neurobiol. 2019;175:54–76.
Gotz J, Halliday G, Nisbet RM. Molecular Pathogenesis of the Tauopathies. Annu Rev Pathol. 2019;14:239–61.
Harada A, Oguchi K, Okabe S, Kuno J, Terada S, Ohshima T, et al. Altered microtubule organization in small-calibre axons of mice lacking tau protein. Nature. 1994;369:488–91.
Takei Y, Teng J, Harada A, Hirokawa N. Defects in axonal elongation and neuronal migration in mice with disrupted tau and map1b genes. J Cell Biol. 2000;150:989–1000.
Yuan A, Kumar A, Peterhoff CM, Duff KE, Nixon RA. Axonal transport rates in vivo are unaffected by tau deletion or overexpression in mice. J Neurosci. 2008;28:1682–7.
Vossel KA, Zhang K, Brodbeck J, Daub AC, Sharma P, Finkbeiner S, et al. Tau Reduction Prevents A{beta}-Induced Defects in Axonal Transport. Science. 2010;330:198.
Lei P, Ayton S, Finkelstein DI, Spoerri L, Ciccotosto GD, Wright DK, et al. Tau deficiency induces parkinsonism with dementia by impairing APP-mediated iron export. Nat Med. 2012;18:291–5.
Lei P, Ayton S, Moon S, Zhang Q, Volitakis I, Finkelstein DI, et al. Motor and cognitive deficits in aged tau knockout mice in two background strains. Mol Neurodegener. 2014;9:29.
Ma QL, Zuo X, Yang F, Ubeda OJ, Gant DJ, Alaverdyan M, et al. Loss of MAP function leads to hippocampal synapse loss and deficits in the Morris Water Maze with aging. J Neurosci. 2014;34:7124–36.
Lei P, Ayton S, Appukuttan AT, Moon S, Duce JA, Volitakis I, et al. Lithium suppression of tau induces brain iron accumulation and neurodegeneration. Mol Psychiatry. 2017;22:396–406.
Ashraf GM, DasGupta D, Alam MZ, Baeesa SS, Alghamdi BS, Anwar F, et al. Inhibition of Microtubule Affinity Regulating Kinase 4 by Metformin: Exploring the Neuroprotective Potential of Antidiabetic Drug through Spectroscopic and Computational Approaches. Molecules. 2022;27:4652.
Bucht G, Adolfsson R, Lithner F, Winblad B. Changes in blood glucose and insulin secretion in patients with senile dementia of Alzheimer type. Acta Med Scand. 1983;213:387–92.
Freude S, Plum L, Schnitker J, Leeser U, Udelhoven M, Krone W, et al. Peripheral hyperinsulinemia promotes tau phosphorylation in vivo. Diabetes. 2005;54:3343–8.
Clodfelder-Miller BJ, Zmijewska AA, Johnson GV, Jope RS. Tau is hyperphosphorylated at multiple sites in mouse brain in vivo after streptozotocin-induced insulin deficiency. Diabetes. 2006;55:3320–5.
Planel E, Tatebayashi Y, Miyasaka T, Liu L, Wang L, Herman M, et al. Insulin dysfunction induces in vivo tau hyperphosphorylation through distinct mechanisms. J Neurosci. 2007;27:13635–48.
Sima AA, Zhang W, Xu G, Sugimoto K, Guberski D, Yorek MA. A comparison of diabetic polyneuropathy in type II diabetic BBZDR/Wor rats and in type I diabetic BB/Wor rats. Diabetologia. 2000;43:786–93.
Yang Y, Ma D, Wang Y, Jiang T, Hu S, Zhang M, et al. Intranasal insulin ameliorates tau hyperphosphorylation in a rat model of type 2 diabetes. J Alzheimer’s Dis. 2013;33:329–38.
Gasparini L, Netzer WJ, Greengard P, Xu H. Does insulin dysfunction play a role in Alzheimer’s disease? Trends Pharmacol Sci. 2002;23:288–93.
Zhu X, Perry G, Smith MA. Insulin signaling, diabetes mellitus and risk of Alzheimer disease. J Alzheimer’s Dis. 2005;7:81–84.
Anderson NJ, King MR, Delbruck L, Jolivalt CG. Role of insulin signaling impairment, adiponectin and dyslipidemia in peripheral and central neuropathy in mice. Disease Model Mech. 2014;7:625–33.
Revill P, Moral MA, Prous JR. Impaired insulin signaling and the pathogenesis of Alzheimer’s disease. Drugs Today. 2006;42:785–90.
Delikkaya B, Moriel N, Tong M, Gallucci G, de la Monte SM. Altered expression of insulin-degrading enzyme and regulator of calcineurin in the rat intracerebral streptozotocin model and human apolipoprotein E-ε4-associated Alzheimer’s disease. Alzheimer’s Dement. 2019;11:392–404.
Nguyen TT, Ta QTH, Nguyen TKO, Nguyen TTD, Giau VV. Type 3 Diabetes and Its Role Implications in Alzheimer’s Disease. Int J Mol Sci. 2020;21:3165.
Chatterjee S, Ambegaokar SS, Jackson GR, Mudher A. Insulin-Mediated Changes in Tau Hyperphosphorylation and Autophagy in a Drosophila Model of Tauopathy and Neuroblastoma Cells. Front Neurosci. 2019;13:801.
Gonçalves RA, Wijesekara N, Fraser PE, De Felice FG. The Link Between Tau and Insulin Signaling: Implications for Alzheimer’s Disease and Other Tauopathies. Front Cell Neurosci. 2019;13:17.
Hong M, Lee VM. Insulin and insulin-like growth factor-1 regulate tau phosphorylation in cultured human neurons. J Biol Chem. 1997;272:19547–53.
Lesort M, Jope RS, Johnson GV. Insulin transiently increases tau phosphorylation: involvement of glycogen synthase kinase-3beta and Fyn tyrosine kinase. J Neurochem. 1999;72:576–84.
Ho KH, Yang X, Osipovich AB, Cabrera O, Hayashi ML, Magnuson MA, et al. Glucose Regulates Microtubule Disassembly and the Dose of Insulin Secretion via Tau Phosphorylation. Diabetes. 2020;69:1936–47.
Barini E, Antico O, Zhao Y, Asta F, Tucci V, Catelani T, et al. Metformin promotes tau aggregation and exacerbates abnormal behavior in a mouse model of tauopathy. Mol Neurodegener. 2016;11:16.
Hull C, Dekeryte R, Koss DJ, Crouch B, Buchanan H, Delibegovic M, et al. Knock-in of Mutated hTAU Causes Insulin Resistance, Inflammation and Proteostasis Disturbance in a Mouse Model of Frontotemporal Dementia. Mol Neurobiol. 2020;57:539–50.
Benderradji H, Kraiem S, Courty E, Eddarkaoui S, Bourouh C, Faivre E, et al. Impaired Glucose Homeostasis in a Tau Knock-In Mouse Model. Front Mol Neurosci. 2022;15:841892.
Abbondante S, Baglietto-Vargas D, Rodriguez-Ortiz CJ, Estrada-Hernandez T, Medeiros R, Laferla FM. Genetic ablation of tau mitigates cognitive impairment induced by type 1 diabetes. Am J Pathol. 2014;184:819–26.
Takalo M, Haapasalo A, Martiskainen H, Kurkinen KM, Koivisto H, Miettinen P, et al. High-fat diet increases tau expression in the brain of T2DM and AD mice independently of peripheral metabolic status. J Nutr Biochem. 2014;25:634–41.
van der Harg JM, Eggels L, Bangel FN, Ruigrok SR, Zwart R, Hoozemans JJM, et al. Insulin deficiency results in reversible protein kinase A activation and tau phosphorylation. Neurobiol Dis. 2017;103:163–73.
Rodriguez-Rodriguez P, Sandebring-Matton A, Merino-Serrais P, Parrado-Fernandez C, Rabano A, Winblad B, et al. Tau hyperphosphorylation induces oligomeric insulin accumulation and insulin resistance in neurons. Brain. 2017;140:3269–85.
Wesseling H, Mair W, Kumar M, Schlaffner CN, Tang S, Beerepoot P, et al. Tau PTM Profiles Identify Patient Heterogeneity and Stages of Alzheimer’s Disease. Cell. 2020;183:1699–1713 e1613.
Zhukareva V, Sundarraj S, Mann D, Sjogren M, Blenow K, Clark CM, et al. Selective reduction of soluble tau proteins in sporadic and familial frontotemporal dementias: an international follow-up study. Acta Neuropathologica. 2003;105:469–76.
Acknowledgements
This work is dedicated to the memory of Dr. Huaxi Xu, who passed away while this paper was being prepared. Huaxi critically reviewed the manuscript. We thank Dr. Linda Partridge (Max Planck Institute for Biology of Ageing) for the kind gift of the dTauKO line, and thank all organ donors and their families for their generosity and for enabling this work. Thanks to the staff of St Vincent’s Institute involved in the islet isolation program and Donatelife for obtaining research consent and providing the Human Pancreata. We also thank Dr. Shuting Zhang for helping with the EOAD sample collection. This work was supported by the National Key Research and Development Project of China (2021YFC2500100), the National Natural Science Foundation of China (81722016, 82071191), the program of the National Clinical Research Center for Geriatrics of West China Hospital (Z2021LC001), the Australian National Health & Medical Research Council (NHMRC), and the Alzheimer’s Australia Dementia Research Foundation. The Florey Institute of Neuroscience and Mental Health and the St. Vincent’s Institute acknowledge support from the Victorian Government, in particular, funding from the Operational Infrastructure Support Grant.
Author information
Authors and Affiliations
Contributions
PL and SPM conceived the project; PL, AIB, and SA raised funds and supervised the overall project; XLL and QW collected clinical samples and performed the biomarker tests. SPM, QZT, XLL, SA, CNJ, BJL, QW, and DRL performed in vivo studies with supervision from PL, AIB, and SA; HET, CK, and TL provided human T2D samples and performed the staining; XLL, CH, JYC, and CHY performed in vitro studies with supervision from PL and SA; XZ performed in Drosophila studies with supervision from HHH; YL and XLD performed and analyzed protein mass spectrometry studies with supervision from LZD; XLL prepared CRISPR-related experiments with supervision from BD and PL; SPM, QZT, SA, AIB, and PL integrated the data and wrote the drafts of the manuscript; all authors edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
AIB is a shareholder in Alterity Ltd, Cogstate Ltd, Eucalyptus Pty Ltd, and Mesoblast Ltd. He is a paid consultant for and has a profit share interest in, Collaborative Medicinal Development Pty Ltd. All other authors declare that they have no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mangiafico, S.P., Tuo, QZ., Li, XL. et al. Tau suppresses microtubule-regulated pancreatic insulin secretion. Mol Psychiatry 28, 3982–3993 (2023). https://doi.org/10.1038/s41380-023-02267-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-023-02267-w
This article is cited by
-
Tau in the pancreas: understanding the link between type 2 diabetes mellitus and Alzheimer’s disease
Signal Transduction and Targeted Therapy (2023)