Abstract
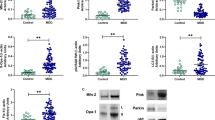
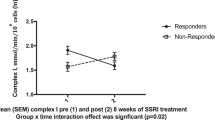
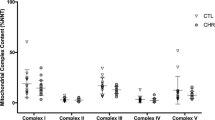
Although mitochondrial dysfunction is known to play an essential role in the pathophysiology of bipolar disorder (BD), there is a glaring gap in our understanding of how mitochondrial dysfunction can modulate clinical phenotypes. An emerging paradigm suggests mitochondria play an important non-energetic role in adaptation to stress, impacting cellular resilience and acting as a source of systemic allostatic load. Known as mitochondrial allostatic load, this (phenomenon) occurs when mitochondria are unable to recalibrate and maintain cell homeostasis. This study aimed to evaluate the composite mitochondrial health index (MHI) in BD subjects and non-psychiatry controls. We will also explore whether lower MIH will be related to higher cell-free mtDNA (ccf-mtDNA) levels and poor clinical outcomes. In this study, 14 BD-I patients and 16 age- and sex-matched non-psychiatry controls were enrolled. Peripheral blood mononuclear cells (PBMCs) were used to measure the enzymatic activities of citrate synthase and complexes I, II, and IV and mtDNA copy number. Ccf-mtDNA was evaluated by qPCR in plasma. Mitochondrial quality control (MQC) proteins were evaluated by western blotting. After adjusting for confounding variables, such as age, sex, body mass index (BMI), and smoking status, patients with BD presented lower MHI compared to non-psychiatry controls, as well as higher ccf-mtDNA levels that negatively correlated with MHI. Because the MQC network is essential to maintain mitochondrial health, MHI and ccf-mtDNA were also examined in relation to several MQC-related proteins, such as Fis-1, Opa-1, and LC3. Our results showed that MHI correlated negatively with Fis-1 and positively with Opa-1 and LC3. Accordingly, ccf-mtDNA had a positive correlation with Fis-1 and a negative correlation with Opa-1 and LC3. Furthermore, we found a noteworthy inverse correlation between illness severity and MHI, with lower MHI and higher ccf-mtDNA levels in subjects with a longer illness duration, worse functional status, and higher depressive symptoms. Our findings indicate that mitochondrial allostatic load contributes to BD, suggesting mitochondria represent a potential biological intersection point that could contribute to impaired cellular resilience and increased vulnerability to stress and mood episodes. Ultimately, by linking mitochondrial dysfunction to disease progression and poor outcomes, we might be able to build a predictive marker that explains how mitochondrial function and its regulation contribute to BD development and that may eventually serve as a treatment guide for both old and new therapeutic targets.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Grande I, Berk M, Birmaher B, Vieta E. Bipolar disorder. Lancet. 2016;387:1561–72.
Kuperberg M, Greenebaum SLA, Nierenberg AA. Targeting mitochondrial dysfunction for bipolar disorder. Curr Top Behav Neurosci. 2021;48:61–99.
Szczepankiewicz A. Evidence for single nucleotide polymorphisms and their association with bipolar disorder. Neuropsychiatr Dis Treat. 2013;9:1573–82.
Scaini G, Andrews T, Lima CNC, Benevenuto D, Streck EL, Quevedo J. Mitochondrial dysfunction as a critical event in the pathophysiology of bipolar disorder. Mitochondrion. 2020;57:23–36.
Scaini G, Andrews T, Lima CNC, Benevenuto D, Streck EL, Quevedo J. Mitochondrial dysfunction as a critical event in the pathophysiology of bipolar disorder. Mitochondrion. 2021;57:23–36.
Kim HK, Chen W, Andreazza AC. The potential role of the NLRP3 inflammasome as a link between mitochondrial complex I dysfunction and inflammation in bipolar disorder. Neural Plast. 2015;2015:408136.
Andreazza AC, Shao L, Wang JF, Young LT. Mitochondrial complex I activity and oxidative damage to mitochondrial proteins in the prefrontal cortex of patients with bipolar disorder. Arch Gen Psychiatry. 2010;67:360–8.
Konradi C, Eaton M, MacDonald ML, Walsh J, Benes FM, Heckers S. Molecular evidence for mitochondrial dysfunction in bipolar disorder. Arch Gen Psychiatry. 2004;61:300–8.
Sun X, Wang JF, Tseng M, Young LT. Downregulation in components of the mitochondrial electron transport chain in the postmortem frontal cortex of subjects with bipolar disorder. J Psychiatry Neurosci. 2006;31:189–96.
Yoshimi N, Futamura T, Bergen SE, Iwayama Y, Ishima T, Sellgren C, et al. Cerebrospinal fluid metabolomics identifies a key role of isocitrate dehydrogenase in bipolar disorder: evidence in support of mitochondrial dysfunction hypothesis. Mol Psychiatry. 2016;21:1504–10.
McMahon FJ, Stine OC, Meyers DA, Simpson SG, DePaulo JR. Patterns of maternal transmission in bipolar affective disorder. Am J Hum Genet. 1995;56:1277–86.
Kato T, Kunugi H, Nanko S, Kato N. Mitochondrial DNA polymorphisms in bipolar disorder. J Affect Disord. 2001;62:151–64.
Colasanti A, Bugiardini E, Amawi S, Poole OV, Skorupinska I, Skorupinska M, et al. Primary mitochondrial diseases increase susceptibility to bipolar affective disorder. J Neurol Neurosurg Psychiatry. 2020;91:892–4.
Picard M, McEwen BS. Psychological stress and mitochondria: a conceptual framework. Psychosom Med. 2018;80:126–40.
Picard M, McEwen BS, Epel ES, Sandi C. An energetic view of stress: focus on mitochondria. Front Neuroendocrinol. 2018;49:72–85.
Bobba-Alves N, Juster RP, Picard M. The energetic cost of allostasis and allostatic load. Psychoneuroendocrinology. 2022;146:105951.
Picard M, Juster RP, McEwen BS. Mitochondrial allostatic load puts the ‘gluc’ back in glucocorticoids. Nat Rev Endocrinol. 2014;10:303–10.
Picard M, Prather AA, Puterman E, Cuillerier A, Coccia M, Aschbacher K, et al. A mitochondrial health index sensitive to mood and caregiving stress. Biol Psychiatry. 2018;84:9–17.
Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–91.
Serdar CC, Cihan M, Yucel D, Serdar MA. Sample size, power and effect size revisited: simplified and practical approaches in pre-clinical, clinical and laboratory studies. Biochem Med. 2021;31:010502.
Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59:22–33.
Williams JB, Kobak KA. Development and reliability of a structured interview guide for the Montgomery Asberg Depression Rating Scale (SIGMA). Br J Psychiatry. 2008;192:52–8.
Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–35.
American Psychiatric Association. Diagnostic and statistical manual of mental disorders, text revision (DSM-IV-TR). 4th ed. Washington, DC, USA: American Psychiatric Association; 2000.
Rosa AR, Sanchez-Moreno J, Martinez-Aran A, Salamero M, Torrent C, Reinares M, et al. Validity and reliability of the Functioning Assessment Short Test (FAST) in bipolar disorder. Clin Pract Epidemiol Ment Health. 2007;3:5.
Srere PA. Citrate synthase: [EC 4.1.3.7. Citrate oxaloacetate-lyase (CoAacetylating)]. Methods Enzymol. 1969;13:3–11.
Cassina A, Radi R. Differential inhibitory action of nitric oxide and peroxynitrite on mitochondrial electron transport. Arch Biochem Biophys. 1996;328:309–16.
Fischer JC, Ruitenbeek W, Berden JA, Trijbels JM, Veerkamp JH, Stadhouders AM, et al. Differential investigation of the capacity of succinate oxidation in human skeletal muscle. Clin Chim Acta. 1985;153:23–36.
Rustin P, Chretien D, Bourgeron T, Gerard B, Rotig A, Saudubray JM, et al. Biochemical and molecular investigations in respiratory chain deficiencies. Clin Chim Acta. 1994;228:35–51.
Tyrka AR, Parade SH, Price LH, Kao HT, Porton B, Philip NS, et al. Alterations of mitochondrial DNA copy number and telomere length with early adversity and psychopathology. Biol Psychiatry. 2016;79:78–86.
Rausser S, Trumpff C, McGill MA, Junker A, Wang W, Ho S, et al. Mitochondrial phenotypes in purified human immune cell subtypes and cell mixtures. bioRxiv. 2021. https://doi.org/10.1101/2020.10.16.342923.
Pyle A, Brennan R, Kurzawa-Akanbi M, Yarnall A, Thouin A, Mollenhauer B, et al. Reduced cerebrospinal fluid mitochondrial DNA is a biomarker for early-stage Parkinson’s disease. Ann Neurol. 2015;78:1000–4.
Demarest TG, McCarthy MM. Sex differences in mitochondrial (dys)function: implications for neuroprotection. J Bioenerg Biomembr. 2015;47:173–88.
Scaini G, Maggi DD, De-Nes BT, Goncalves CL, Ferreira GK, Teodorak BP, et al. Activity of mitochondrial respiratory chain is increased by chronic administration of antidepressants. Acta Neuropsychiatr. 2011;23:112–8.
Chistiakov DA, Sobenin IA, Revin VV, Orekhov AN, Bobryshev YV. Mitochondrial aging and age-related dysfunction of mitochondria. Biomed Res Int. 2014;2014:238463.
Scaini G, Fries GR, Valvassori SS, Zeni CP, Zunta-Soares G, Berk M, et al. Perturbations in the apoptotic pathway and mitochondrial network dynamics in peripheral blood mononuclear cells from bipolar disorder patients. Transl Psychiatry. 2017;7:e1111.
Scaini G, Barichello T, Fries GR, Kennon EA, Andrews T, Nix BR, et al. TSPO upregulation in bipolar disorder and concomitant downregulation of mitophagic proteins and NLRP3 inflammasome activation. Neuropsychopharmacology. 2019;44:1291–9.
Scaini G, Valvassori SS, Diaz AP, Lima CN, Benevenuto D, Fries GR, et al. Neurobiology of bipolar disorders: a review of genetic components, signaling pathways, biochemical changes, and neuroimaging findings. Braz J Psychiatry. 2020;42:536–51.
Ashrafi G, Schwarz TL. The pathways of mitophagy for quality control and clearance of mitochondria. Cell Death Differ. 2013;20:31–42.
McBride HM, Neuspiel M, Wasiak S. Mitochondria: more than just a powerhouse. Curr Biol. 2006;16:R551–60.
Busch KB, Kowald A, Spelbrink JN. Quality matters: how does mitochondrial network dynamics and quality control impact on mtDNA integrity? Philos Trans R Soc Lond B Biol Sci. 2014;369:20130442.
Yang L, Long Q, Liu J, Tang H, Li Y, Bao F, et al. Mitochondrial fusion provides an ‘initial metabolic complementation’ controlled by mtDNA. Cell Mol Life Sci. 2015;72:2585–98.
Zhang Q, Itagaki K, Hauser CJ. Mitochondrial DNA is released by shock and activates neutrophils via p38 map kinase. Shock. 2010;34:55–9.
Nakahira K, Haspel JA, Rathinam VA, Lee SJ, Dolinay T, Lam HC, et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol. 2011;12:222–30.
Pinti M, Cevenini E, Nasi M, De Biasi S, Salvioli S, Monti D, et al. Circulating mitochondrial DNA increases with age and is a familiar trait: implications for “inflamm-aging”. Eur J Immunol. 2014;44:1552–62.
Wu G, Zhu Q, Zeng J, Gu X, Miao Y, Xu W, et al. Extracellular mitochondrial DNA promote NLRP3 inflammasome activation and induce acute lung injury through TLR9 and NF-kappaB. J Thorac Dis. 2019;11:4816–28.
Yuzefovych LV, Pastukh VM, Ruchko MV, Simmons JD, Richards WO, Rachek LI. Plasma mitochondrial DNA is elevated in obese type 2 diabetes mellitus patients and correlates positively with insulin resistance. PLoS ONE. 2019;14:e0222278.
Fries GR, Walss-Bass C, Bauer ME, Teixeira AL. Revisiting inflammation in bipolar disorder. Pharmacol, Biochem Behav. 2019;177:12–9.
Grande I, Magalhaes PV, Kunz M, Vieta E, Kapczinski F. Mediators of allostasis and systemic toxicity in bipolar disorder. Physiol Behav. 2012;106:46–50.
Juster RP, Russell JJ, Almeida D, Picard M. Allostatic load and comorbidities: a mitochondrial, epigenetic, and evolutionary perspective. Dev Psychopathol. 2016;28:1117–46.
Magalhaes PV, Dean OM, Bush AI, Copolov DL, Weisinger D, Malhi GS, et al. Systemic illness moderates the impact of N-acetyl cysteine in bipolar disorder. Prog Neuro Psychopharmacol Biol Psychiatry. 2012;37:132–5.
Cuperfain AB, Kennedy JL, Goncalves VF. Overlapping mechanisms linking insulin resistance with cognition and neuroprogression in bipolar disorder. Neurosci Biobehav Rev. 2020;111:125–34.
Grewal S, McKinlay S, Kapczinski F, Pfaffenseller B, Wollenhaupt-Aguiar B. Biomarkers of neuroprogression and late staging in bipolar disorder: a systematic review. Aust NZ J Psychiatry. 2023;57:328–43.
Kapczinski NS, Mwangi B, Cassidy RM, Librenza-Garcia D, Bermudez MB, Kauer-Sant’anna M, et al. Neuroprogression and illness trajectories in bipolar disorder. Expert Rev Neurother. 2017;17:277–85.
Sanchez-Moreno J, Martinez-Aran A, Tabares-Seisdedos R, Torrent C, Vieta E, Ayuso-Mateos JL. Functioning and disability in bipolar disorder: an extensive review. Psychother Psychosom. 2009;78:285–97.
Sartori JM, Reckziegel R, Passos IC, Czepielewski LS, Fijtman A, Sodre LA, et al. Volumetric brain magnetic resonance imaging predicts functioning in bipolar disorder: a machine learning approach. J Psychiatr Res. 2018;103:237–43.
Rosa AR, Reinares M, Franco C, Comes M, Torrent C, Sanchez-Moreno J, et al. Clinical predictors of functional outcome of bipolar patients in remission. Bipolar Disord. 2009;11:401–9.
Reyes AN, Cardoso TA, Jansen K, Mondin TC, Souza LDM, Magalhaes PVS, et al. Functional impairment and cognitive performance in mood disorders: a community sample of young adults. Psychiatry Res. 2017;251:85–9.
Rosa AR, Magalhaes PV, Czepielewski L, Sulzbach MV, Goi PD, Vieta E, et al. Clinical staging in bipolar disorder: focus on cognition and functioning. J Clin Psychiatry. 2014;75:e450–6.
Funding
The research funding support from the University of Texas Health Science Center at Houston, the Louis A. Faillace, MD Endowment Funds, and the Translational Psychiatry Program to GS, GRF, and JQ are acknowledged. The Translational Psychiatry Program (USA) is funded by the Department of Psychiatry and Behavioral Sciences, McGovern Medical School at UTHealth, and Linda Gail Behavioral Health Research Fund. Center of Excellence on Mood Disorders (USA) is funded by the Pat Rutherford Jr. Chair in Psychiatry, the John S. Dunn Foundation, and the Anne and Don Fizer Foundation Endowment for Depression Research. Translational Psychiatry Laboratory (Brazil) is funded by grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundação de Amparo à Pesquisa e Inovação do Estado de Santa Catarina (FAPESC), Instituto Cérebro e Mente and the University of Southern Santa Catarina (UNESC). GRF is funded by the National Institute of Mental Health (5K01MH121580) and the Baszucki Research Foundation.
Author information
Authors and Affiliations
Contributions
GS conceived and designed the study hypothesis. RCC and GS analyzed and interpreted the data, conducted literature searches, prepared tables/figures, and co-wrote the first draft of the manuscript. CNCL assisted with the biochemical assessments, and GRF assisted with the statistical analysis and data interpretation. JQ, GZS, and JCS were involved in subject recruitment, data collection, and clinical data interpretation. All authors reviewed the manuscript for intellectual content and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cordeiro, R.C., Lima, C.N.C., Fries, G.R. et al. Mitochondrial health index correlates with plasma circulating cell-free mitochondrial DNA in bipolar disorder. Mol Psychiatry 28, 4622–4631 (2023). https://doi.org/10.1038/s41380-023-02249-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-023-02249-y