Abstract
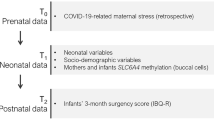
Antenatal exposures to maternal stress and to particulate matter with an aerodynamic diameter of less than 2.5 μm (PM2.5) have been independently associated with developmental outcomes in early infancy and beyond. Knowledge about their joint impact, biological mechanisms of their effects and timing-effects, is still limited. Both PM2.5 and maternal stress exposure during pregnancy might result in altered patterns of DNA methylation in specific stress-related genes, such as the serotonin transporter gene (SLC6A4 DNAm), that might, in turn, influence infant development across several domains, including bio-behavioral, cognitive and socio-emotional domains. Here, we investigated the independent and interactive influence of variations in antenatal exposures to maternal pandemic-related stress (PRS) and PM2.5 on SLC6A4 DNAm levels in newborns. Mother–infant dyads (N = 307) were enrolled at delivery during the COVID-19 pandemic. Infants’ methylation status was assessed in 13 CpG sites within the SLC6A4 gene’s region (chr17:28562750–28562958) in buccal cells at birth and women retrospectively report on PRS. PM2.5 exposure throughout the entire gestation and at each gestational trimester was estimated using a spatiotemporal model based on residential address. Among several potentially confounding socio-demographic and health-related factors, infant’s sex was significantly associated with infants’ SLC6A4 DNAm levels, thus hierarchical regression models were adjusted for infant’s sex. Higher levels of SLC6A4 DNAm at 6 CpG sites were found in newborns born to mothers reporting higher levels of antenatal PRS and greater PM2.5 exposure across gestation, while adjusting for infant’s sex. These effects were especially evident when exposure to elevated PM2.5 occurred during the second trimester of pregnancy. Several important brain processes (e.g., synaptogenesis and myelination) occur during mid-pregnancy, potentially making the second trimester a sensitive time window for the effects of stress-related exposures. Understanding the interplay between environmental and individual-level stressors has important implications for the improvement of mother-infant health during and after the pandemic.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Grossman AW, Churchill JD, McKinney BC, Kodish IM, Otte SL, Greenough WT. Experience effects on brain development: possible contributions to psychopathology. J Child Psychol Psychiatry. 2003;44:33–63.
Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med. 2008;359:61–73.
Castagna A, Mascheroni E, Fustinoni S, Montirosso R. Air pollution and neurodevelopmental skills in preschool- and school-aged children: a systematic review. Neurosci Biobehav Rev. 2022;136:104623.
Bergh BRH, Van den Heuvel MI, van den Lahti M, Braeken M, de Rooij SR, Entringer S, et al. Prenatal developmental origins of behavior and mental health: the influence of maternal stress in pregnancy. Neurosci Biobehav Rev. 2020;117:26–64.
Kostović I, Sedmak G, Judaš M. Neural histology and neurogenesis of the human fetal and infant brain. Neuroimage. 2019;188:743–73.
Grumi S, Provenzi L, Accorsi P, Biasucci G, Cavallini A, Decembrino L, et al. Depression and anxiety in mothers who were pregnant during the COVID-19 outbreak in Northern Italy: the role of pandemic-related emotional stress and perceived social support. Front Psychiatry. 2021;12:716488.
Tomfohr-Madsen LM, Racine N, Giesbrecht GF, Lebel C, Madigan S. Depression and anxiety in pregnancy during COVID-19: a rapid review and meta-analysis. Psychiatry Res. 2021;300:113912.
Pagès N, Gorgui J, Wang C, Wang X, Zhao JP, Tchuente V, et al. The Impact of COVID-19 on maternal mental health during pregnancy: a comparison between Canada and China within the CONCEPTION Cohort. Int J Environ Res Public Health. 2022;19:12386.
Camoni L, Mirabella F, Gigantesco A, Brescianini S, Ferri M, Palumbo G, et al. The impact of the COVID-19 pandemic on women’s perinatal mental health: preliminary data on the risk of perinatal depression/anxiety from a national survey in Italy. Int J Environ Res Public Health. 2022;19:14822.
Provenzi L, Mambretti F, Villa M, Grumi S, Citterio A, Bertazzoli E, et al. Hidden pandemic: COVID-19-related stress, SLC6A4 methylation, and infants’ temperament at 3 months. Sci Rep. 2021;11:15658.
Provenzi L, Grumi S, Altieri L, Bensi G, Bertazzoli E, Biasucci G et al. Prenatal maternal stress during the COVID-19 pandemic and infant regulatory capacity at 3 months: a longitudinal study. Dev Psychopathol. 2023;35:35–43.
Buthmann JL, Miller JG, Gotlib IH. Maternal-prenatal stress and depression predict infant temperament during the COVID-19 pandemic. Dev Psychopathol. 2022:1–9. https://doi.org/10.1017/S0954579422001055. Online ahead of print.
López-Morales H, Gelpi TR, Del-Valle MV, Canet-Juric L, Biota M, Andrés ML et al. The pandemial babies: effects of maternal stress on temperament of babies gestated and born during the pandemic. Curr Psychol. 2022:1–13. https://doi.org/10.1007/s12144-022-03976-1. Online ahead of print.
Duguay G, Garon-Bissonnette J, Lemieux R, Dubois-Comtois K, Mayrand K, Berthelot N. Socioemotional development in infants of pregnant women during the COVID-19 pandemic: the role of prenatal and postnatal maternal distress. Child Adolesc Psychiatry Ment Health. 2022;16:28.
Nazzari S, Grumi S, Biasucci G, Decembrino L, Fazzi E, Giacchero R, et al. Maternal pandemic-related stress during pregnancy associates with infants’ socio-cognitive development at 12 months: a longitudinal multi-centric study. Plos one. 2023;18:e0284578.
Papadopoulos A, Nichols ES, Mohsenzadeh Y, Giroux I, Mottola MF, Van LRJ, et al. Prenatal and postpartum maternal mental health and neonatal motor outcomes during the COVID-19 pandemic. J Affect Disord Rep. 2022;10:100387.
Manning KY, Long X, Watts D, Tomfohr-Madsen L, Giesbrecht GF, Lebel C. Prenatal maternal distress during the COVID-19 pandemic and associations with infant brain connectivity. Biol Psychiatry. 2022;92:701–8.
de KTM, Driece HA, Hogervorst JG, Briedé JJ. Toxicological assessment of ambient and traffic-related particulate matter: a review of recent studies. Mutat Res. 2006;613:103–22.
Mehta M, Chen LC, Gordon T, Rom W, Tang MS. Particulate matter inhibits DNA repair and enhances mutagenesis. Mutat Res. 2008;657:116–21.
Risom L, Møller P, Loft S. Oxidative stress-induced DNA damage by particulate air pollution. Mutat Res. 2005;592:119–37.
Loftus CT, Hazlehurst MF, Szpiro AA, Ni Y, Tylavsky FA, Bush NR, et al. Prenatal air pollution and childhood IQ: preliminary evidence of effect modification by folate. Environ Res. 2019;176:108505.
Margolis AE, Liu R, Conceição VA, Ramphal B, Pagliaccio D, DeSerisy ML, et al. Convergent neural correlates of prenatal exposure to air pollution and behavioral phenotypes of risk for internalizing and externalizing problems: potential biological and cognitive pathways. Neurosci Biobehav Rev. 2022;137:104645.
Guxens M, Lubczyńska MJ, Muetzel RL, Dalmau-Bueno A, Jaddoe VWV, Hoek G, et al. Air pollution exposure during fetal life, brain morphology, and cognitive function in school-age children. Biol Psychiatry. 2018;84:295–303.
Kundakovic M, Jaric I. The epigenetic link between prenatal adverse environments and neurodevelopmental disorders. Genes. 2017;8:104.
Nazzari S, Frigerio A. The programming role of maternal antenatal inflammation on infants’ early neurodevelopment: a review of human studies: special section on translational and neuroscience studies in affective disorders section editor, Maria Nobile MD, PhD. J Affect Disord. 2020;263:739–46.
Rakers F, Rupprecht S, Dreiling M, Bergmeier C, Witte OW, Schwab M. Transfer of maternal psychosocial stress to the fetus. Neurosci Biobehav Rev. 2017;117:185–97.
Meaney MJ. Epigenetics and the biological definition of gene x environment interactions. Child Dev. 2010;81:41–79.
Provenzi L, Giorda R, Beri S, Montirosso R. SLC6A4 methylation as an epigenetic marker of life adversity exposures in humans: a systematic review of literature. Neurosci Biobehav Rev. 2016;71:7–20.
Dukal H, Frank J, Lang M, Treutlein J, Gilles M, Wolf IA, et al. New-born females show higher stress- and genotype-independent methylation of SLC6A4 than males. Borderline Personal Disord Emot Dysregul. 2015;2:8.
DeLano K, Folger AT, Ding L, Ji H, Yolton K, Ammerman RT, et al. Associations between maternal community deprivation and infant DNA methylation of the SLC6A4 Gene. Front Public Health. 2020;8:557195.
Devlin AM, Brain U, Austin J, Oberlander TF. Prenatal exposure to maternal depressed mood and the MTHFR C677T variant affect SLC6A4 methylation in infants at birth. PLoS One. 2010;5:e12201.
Wankerl M, Miller R, Kirschbaum C, Hennig J, Stalder T, Alexander N. Effects of genetic and early environmental risk factors for depression on serotonin transporter expression and methylation profiles. Transl Psychiatry. 2014;4:e402.
Nazzari S, Grumi S, Mambretti F, Villa M, Giorda R, Provenzi L. Maternal and infant NR3C1 and SLC6A4 epigenetic signatures of the COVID-19 pandemic lockdown: when timing matters. Transl Psychiatry. 2022;12:386.
Gruzieva O, Xu CJ, Yousefi P, Relton C, Merid SK, Breton CV, et al. Prenatal particulate air pollution and DNA methylation in newborns: an epigenome-wide meta-analysis. Environ Health Perspect. 2019;127:57012.
Breton CV, Gao L, Yao J, Siegmund KD, Lurmann F, Gilliland F. Particulate matter, the newborn methylome, and cardio-respiratory health outcomes in childhood. Environ Epigenet. 2016;2:dvw005.
Bové H, Bongaerts E, Slenders E, Bijnens EM, Saenen ND, Gyselaers W, et al. Ambient black carbon particles reach the fetal side of human placenta. Nat Commun. 2019;10:3866.
Liu NM, Miyashita L, Maher BA, McPhail G, Jones CJP, Barratt B, et al. Evidence for the presence of air pollution nanoparticles in placental tissue cells. Sci Total Environ. 2021;751:142235.
Ghazi T, Naidoo P, Naidoo RN, Chuturgoon AA. Prenatal air pollution exposure and placental DNA methylation changes: implications on fetal development and future disease susceptibility. Cell. 2021;10:3025.
Janssen BG, Byun HM, Gyselaers W, Lefebvre W, Baccarelli AA, Nawrot TS. Placental mitochondrial methylation and exposure to airborne particulate matter in the early life environment: an ENVIRONAGE birth cohort study. Epigenetics. 2015;10:536–44.
Maghbooli Z, Hossein-Nezhad A, Adabi E, Asadollah-Pour E, Sadeghi M, Mohammad-Nabi S, et al. Air pollution during pregnancy and placental adaptation in the levels of global DNA methylation. PLoS One. 2018;13:e0199772.
Janssen BG, Godderis L, Pieters N, Poels K, Kiciński M, Cuypers A, et al. Placental DNA hypomethylation in association with particulate air pollution in early life. Part Fibre Toxicol. 2013;10:22.
Taylor SE. Mechanisms linking early life stress to adult health outcomes. Proc Natl Acad Sci USA. 2010;107:8507–12.
Dufford AJ, Spann M, Scheinost D. How prenatal exposures shape the infant brain: insights from infant neuroimaging studies. Neurosci Biobehav Rev. 2021;131:47–58.
Bishop DV. Genes, cognition, and communication: insights from neurodevelopmental disorders. Ann NY Acad Sci. 2009;1156:1–18.
Bayer TA, Falkai P, Maier W. Genetic and non-genetic vulnerability factors in schizophrenia: the basis of the two hit hypothesis. J Psychiatr Res. 1999;33:543–8.
Nederhof E, Schmidt MV. Mismatch or cumulative stress: toward an integrated hypothesis of programming effects. Physiol Behav. 2012;106:691–700.
Padula AM, Rivera-Núñez Z, Barrett ES. Combined impacts of prenatal environmental exposures and psychosocial stress on offspring health: air pollution and metals. Curr Environ Health Rep. 2020;7:89–100.
Erickson AC, Ostry A, Chan HM, Arbour L. Air pollution, neighborhood and maternal-level factors modify the effect of smoking on birth weight: a multilevel analysis in British Columbia, Canada. BMC Public Health. 2016;16:585.
Lee A, Leon HHH, Mathilda CYH, Bose S, Rosa MJ, Kloog I, et al. Prenatal fine particulate exposure and early childhood asthma: effect of maternal stress and fetal sex. J Allergy Clin Immunol. 2018;141:1880–6.
Rosa MJ, Just AC, Kloog I, Pantic I, Schnaas L, Lee A, et al. Prenatal particulate matter exposure and wheeze in Mexican children: effect modification by prenatal psychosocial stress. Ann Allergy Asthma Immunol. 2017;119:232–7.e1.
Provenzi L, Grumi S, Giorda R, Biasucci G, Bonini R, Cavallini A, et al. Measuring the Outcomes of Maternal COVID-19-related Prenatal Exposure (MOM-COPE): study protocol for a multicentric longitudinal project. BMJ Open. 2020;10:e044585.
Provenzi L, Fumagalli M, Scotto di MG, Giorda R, Morandi F, Sirgiovanni I, et al. Pain-related increase in serotonin transporter gene methylation associates with emotional regulation in 4.5-year-old preterm-born children. Acta Paediatr. 2020;109:1166–74.
Cecil CA, Lysenko LJ, Jaffee SR, Pingault JB, Smith RG, Relton CL, et al. Environmental risk, Oxytocin Receptor Gene (OXTR) methylation and youth callous-unemotional traits: a 13-year longitudinal study. Mol Psychiatry. 2014;19:1071–7.
Checknita D, Bendre M, Ekström TJ, Comasco E, Tiihonen J, Hodgins S, et al. Monoamine oxidase a genotype and methylation moderate the association of maltreatment and aggressive behaviour. Behav Brain Res. 2020;382:112476.
Nazzari S, Grumi S, Villa M, Mambretti F, Biasucci G, Decembrino L, et al. Sex-dependent association between variability in infants’ OXTR methylation at birth and negative affectivity at 3 months. Psychoneuroendocrinology. 2022;145:105920.
O’Donnell KJ, Meaney MJ. Fetal origins of mental health: the developmental origins of health and disease hypothesis. Am J Psychiatry. 2017;174:319–28.
Power MC, Kioumourtzoglou MA, Hart JE, Okereke OI, Laden F, Weisskopf MG. The relation between past exposure to fine particulate air pollution and prevalent anxiety: observational cohort study. BMJ. 2015;350:h1111.
Mehta AJ, Kubzansky LD, Coull BA, Kloog I, Koutrakis P, Sparrow D, et al. Associations between air pollution and perceived stress: the veterans administration normative aging study. Environ Health. 2015;14:10.
Lin Y, Zhou L, Xu J, Luo Z, Kan H, Zhang J, et al. The impacts of air pollution on maternal stress during pregnancy. Sci Rep. 2017;7:1–11.
Thomson EM. Air pollution, stress, and allostatic load: linking systemic and central nervous system impacts. J Alzheimers Dis. 2019;69:597–614.
Frodl T, Szyf M, Carballedo A, Ly V, Dymov S, Vaisheva F, et al. DNA methylation of the serotonin transporter gene (SLC6A4) is associated with brain function involved in processing emotional stimuli. J Psychiatry Neurosci. 2015;40:296–305.
Booij L, Szyf M, Carballedo A, Frey EM, Morris D, Dymov S, et al. DNA methylation of the serotonin transporter gene in peripheral cells and stress-related changes in hippocampal volume: a study in depressed patients and healthy controls. PLoS One. 2015;10:e0119061.
Alexander N, Wankerl M, Hennig J, Miller R, Zankert S, Steudte-Schmiedgen S, et al. DNA methylation profiles within the serotonin transporter gene moderate the association of 5-HTTLPR and cortisol stress reactivity. Transl Psychiatry. 2014;4:e443.
Sugawara H, Bundo M, Ishigooka J, Iwamoto K, Kato T. Epigenetic regulation of serotonin transporter in psychiatric disorders. J Genet Genom. 2013;40:325–9.
Southerland VA, Brauer M, Mohegh A, Hammer MS, van Donkelaar A, Martin RV, et al. Global urban temporal trends in fine particulate matter (PM(2·5)) and attributable health burdens: estimates from global datasets. Lancet Planet Health. 2022;6:e139–46.
Cohen AJ, Brauer M, Burnett R, Anderson HR, Frostad J, Estep K, et al. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the Global Burden of Diseases Study 2015. Lancet. 2017;389:1907–18.
Parikh MN, Brokamp C, Rasnick E, Ding L, Mersha TB, Bowers K, et al. Epigenome-wide association of neonatal methylation and trimester-specific prenatal PM(2.5) exposure. Environ Epidemiol. 2022;6:e227.
Park J, Kim WJ, Kim J, Jeong CY, Park H, Hong YC, et al. Prenatal exposure to traffic-related air pollution and the DNA methylation in cord blood cells: MOCEH study. Int J Environ Res Public Health. 2022;19:3292.
Andersen SL. Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci Biobehav Rev. 2003;27:3–18.
Ta GZ, Peterson BS. Normal development of brain circuits. Neuropsychopharmacology. 2010;35:147–68.
Sunyer J, Dadvand P. Pre-natal brain development as a target for urban air pollution. Basic Clin Pharm Toxicol. 2019;125:81–8.
Thompson TM, Sharfi D, Lee M, Yrigollen CM, Naumova OY, Grigorenko EL. Comparison of whole-genome DNA methylation patterns in whole blood, saliva, and lymphoblastoid cell lines. Behav Genet. 2013;43:168–76.
Armstrong DA, Lesseur C, Conradt E, Lester BM, Marsit CJ. Global and gene-specific DNA methylation across multiple tissues in early infancy: implications for children’s health research. FASEB J. 2014;28:2088–97.
Dipietro JA, Costigan KA, Sipsma HL. Continuity in self-report measures of maternal anxiety, stress, and depressive symptoms from pregnancy through two years postpartum. J Psychosom Obstet Gynecol. 2008;29:115–24.
Provenzi L, Brambilla M, Scotto di MG, Montirosso R, Borgatti R. Maternal caregiving and DNA methylation in human infants and children: systematic review. Genes Brain Behav. 2020;19:e12616.
Nazzari S, Fearon P, Rice F, Molteni M, Frigerio A. Maternal caregiving moderates the impact of antenatal maternal cortisol on infant stress regulation. J Child Psychol Psychiatry. 2022;63:871–80.
Frigerio A, Nettuno F, Nazzari S. Maternal mood moderates the trajectory of emotional and behavioural problems from pre- to during the COVID-19 lockdown in preschool children. Eur Child Adolesc Psychiatry. 2023;32:1189–99.
Funding
SN was supported by a co-funding of the European Union—European Social Fund REACT-EU, PON Ricerca e Innovazione 2014–2020. LP was supported by the Italian Health Ministry, Ricerca Corrente 2022–2023. Work supported by #NEXTGENERATIONEU (NGEU) and funded by the Ministry of University and Research (MUR), National Recovery and Resilience Plan (NRRP), project MNESYS (PE0000006) – A Multiscale integrated approach to the study of the nervous system in health and disease (DN. 1553 11.10.2022).
Author information
Authors and Affiliations
Contributions
SN contributed to data analysis and drafting the manuscript; LC contributed to data analysis and conceptualization; SG and JM contributed to study design and data management; EP contributed to data collection and pre-processing of pollution data; GM and RB supervised the methodology and contributed to the conceptualization; RB contributed to data analysis and preprocessing of epigenetic data; LP conceptualized the study, obtained funding, coordinated the project, contributed to data analysis and first manuscript draft. All authors contributed to the final manuscript draft and approved the submission.
Corresponding author
Ethics declarations
Competing interests
The author declares no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nazzari, S., Cagliero, L., Grumi, S. et al. Prenatal exposure to environmental air pollution and psychosocial stress jointly contribute to the epigenetic regulation of the serotonin transporter gene in newborns. Mol Psychiatry 28, 3503–3511 (2023). https://doi.org/10.1038/s41380-023-02206-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-023-02206-9