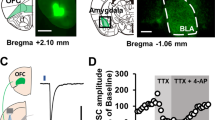
Abstract
Depression is a common and severe mental disorder. Evidence suggested a substantial causal relationship between stressful life events and the onset of episodes of major depression. However, the stress-induced pathogenesis of depression and the related neural circuitry is poorly understood. Here, we investigated how cholecystokinin (CCK) and CCKBR in the basolateral amygdala (BLA) are implicated in stress-mediated depressive-like behavior. The BLA mediates emotional memories, and long-term potentiation (LTP) is widely considered a trace of memory. We identified that the cholecystokinin knockout (CCK-KO) mice impaired LTP in the BLA, while the application of CCK4 induced LTP after low-frequency stimulation (LFS). The entorhinal cortex (EC) CCK neurons project to the BLA and optogenetic activation of EC CCK afferents to BLA-promoted stress susceptibility through the release of CCK. We demonstrated that EC CCK neurons innervate CCKBR cells in the BLA and CCK-B receptor knockout (CCKBR-KO) mice impaired LTP in the BLA. Moreover, the CCKBR antagonists also blocked high-frequency stimulation (HFS) induced LTP formation in the BLA. Notably, CCKBR antagonists infusion into the BLA displayed an antidepressant-like effect in the chronic social defeat stress model. Together, these results indicate that CCKBR could be a potential target to treat depression.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Daly M, Robinson E. Depression and anxiety during COVID-19. Lancet. 2022;399:518.
Ferguson JM. SSRI antidepressant medications: adverse effects and tolerability. Prim Care Companion J Clin psychiatry. 2001;3:22.
Birmes P, Coppin D, Schmitt L, Lauque D. Serotonin syndrome: a brief review. Cmaj. 2003;168:1439–42.
Mitchell AJ. Two-week delay in onset of action of antidepressants: new evidence. Br J Psychiatry. 2006;188:105–6.
Hammen C. Stress and depression. Annu Rev Clin Psychol. 2005;1:293–319.
Kessler RC. The effects of stressful life events on depression. Annu Rev Psychol. 1997;48:191–214.
Kendler KS, Karkowski LM, Prescott CA. Causal relationship between stressful life events and the onset of major depression. Am J Psychiatry. 1999;156:837–41.
Hu H. Reward and aversion. Annu Rev Neurosci. 2016;39:297–324.
Russo SJ, Nestler EJ. The brain reward circuitry in mood disorders. Nat Rev Neurosci. 2013;14:609–25.
Asim M, Hao B, Waris A, Liang Y-M, Wang X-G. Ketamine attenuates the PTSD-like effect via regulation of glutamatergic signaling in the nucleus accumbens of mice. Mol Cell Neurosci. 2022;120:103723.
Asim M, Hao B, Yang Y-H, Fan B-F, Xue L, Shi Y-W, et al. Ketamine alleviates fear generalization through GluN2B-BDNF signaling in mice. Neurosci Bull. 2020;36:153–64.
Asim M, Wang B, Hao B, Wang X. Ketamine for post-traumatic stress disorders and it’s possible therapeutic mechanism. Neurochem Int. 2021;146:105044.
Baxter MG, Murray EA. The amygdala and reward. Nat Rev Neurosci. 2002;3:563–73.
Hamilton JP, Gotlib IH. Neural substrates of increased memory sensitivity for negative stimuli in major depression. Biol Psychiatry. 2008;63:1155–62.
O’Neill P-K, Gore F, Salzman CD. Basolateral amygdala circuitry in positive and negative valence. Curr Opin Neurobiol. 2018;49:175–83.
Asim M, Wang H, Waris A. Altered neurotransmission in stress-induced depressive disorders: The underlying role of the amygdala in depression. Neuropeptides. 2023;98:102322.
Siegle GJ, Thompson W, Carter CS, Steinhauer SR, Thase ME. Increased amygdala and decreased dorsolateral prefrontal BOLD responses in unipolar depression: related and independent features. Biol Psychiatry. 2007;61:198–209.
Peluso MA, Glahn DC, Matsuo K, Monkul ES, Najt P, Zamarripa F, et al. Amygdala hyperactivation in untreated depressed individuals. Psychiatry Res. 2009;173:158–61.
Yang TT, Simmons AN, Matthews SC, Tapert SF, Frank GK, Max JE, et al. Adolescents with major depression demonstrate increased amygdala activation. J Am Acad Child Adolesc Psychiatry. 2010;49:42–51.
Feng H, Su J, Fang W, Chen X, He J. The entorhinal cortex modulates trace fear memory formation and neuroplasticity in the mouse lateral amygdala via cholecystokinin. Elife. 2021;10:e69333.
Fu J-Y, Yu X-D, Zhu Y, Xie S-Z, Tang M-Y, Yu B, et al. Whole-brain map of long-range monosynaptic inputs to different cell types in the amygdala of the mouse. Neurosci Bull. 2020;36:1381–94.
Hafting T, Fyhn M, Molden S, Moser M-B, Moser EI. Microstructure of a spatial map in the entorhinal cortex. Nature. 2005;436:801–6.
Fyhn M, Molden S, Witter MP, Moser EI, Moser M-B. Spatial representation in the entorhinal cortex. Science. 2004;305:1258–64.
Maren S, Fanselow MS. Electrolytic lesions of the fimbria/fornix, dorsal hippocampus, or entorhinal cortex produce anterograde deficits in contextual fear conditioning in rats. Neurobiol Learn Mem. 1997;67:142–9.
Lu J, Zhang Z, Yin X, Tang Y, Ji R, Chen H, et al. An entorhinal-visual cortical circuit regulates depression-like behaviors. Mol Psychiatry. 2022;27:1–14.
Yun S, Reynolds RP, Petrof I, White A, Rivera PD, Segev A, et al. Stimulation of entorhinal cortex–dentate gyrus circuitry is antidepressive. Nat Med. 2018;24:658–66.
Sun W, Wu H, Peng Y, Tang P, Zhao M, Zheng CX, et al. Heterosynaptic Plasticity of the Visuo-auditory Projection Requires Cholecystokinin released from Entorhinal Cortex Afferents. bioRxiv. https://doi.org/10.1101/2022.10.04.510820.
Hernando F, Fuentes J, Roques BP, Ruiz-Gayo M. The CCKB receptor antagonist, L-365,260, elicits antidepressant-type effects in the forced-swim test in mice. Eur J Pharmacol. 1994;261:257–63.
Löfberg C, Ågren H, Harro J, Oreland L. Cholecystokinin in CSF from depressed patients: possible relations to severity of depression and suicidal behaviour. Eur Neuropsychopharmacol. 1998;8:153–7.
Becker C, Zeau B, Rivat C, Blugeot A, Hamon M, Benoliel J. Repeated social defeat-induced depression-like behavioral and biological alterations in rats: involvement of cholecystokinin. Mol Psychiatry. 2008;13:1079–92.
Del Boca C, Lutz P, Le Merrer J, Koebel P, Kieffer B. Cholecystokinin knock-down in the basolateral amygdala has anxiolytic and antidepressant-like effects in mice. Neuroscience. 2012;218:185–95.
Karthik L, Kumar G, Keswani T, Bhattacharyya A, Chandar SS, Rao KB. Protease inhibitors from marine actinobacteria as a potential source for antimalarial compound. PloS One. 2014;9:e90972.
Wank SA. Cholecystokinin receptors. Am J Physiol-Gastrointest Liver Physiol. 1995;269:G628–G646.
Honda T, Wada E, Battey JF, Wank SA. Differential gene expression of CCKA and CCKB receptors in the rat brain. Mol Cell Neurosci. 1993;4:143–54.
Chen X, Li X, Wong YT, Zheng X, Wang H, Peng Y, et al. Cholecystokinin release triggered by NMDA receptors produces LTP and sound–sound associative memory. Proc Natl Acad Sci. 2019;116:6397–406.
Li X, Yu K, Zhang Z, Sun W, Yang Z, Feng J, et al. Cholecystokinin from the entorhinal cortex enables neural plasticity in the auditory cortex. Cell Res. 2014;24:307–30.
Zhang Z, Zheng X, Sun W, Peng Y, Guo Y, Lu D, et al. Visuoauditory associative memory established with cholecystokinin under anesthesia is retrieved in behavioral contexts. J Neurosci. 2020;40:2025–37.
Jing M, Zhang Y, Wang H, Li Y. G‐protein‐coupled receptor‐based sensors for imaging neurochemicals with high sensitivity and specificity. J Neurochem. 2019;151:279–88.
Bruno CA, O’Brien C, Bryant S, Mejaes JI, Estrin DJ, Pizzano C, et al. pMAT: An open-source software suite for the analysis of fiber photometry data. Pharmacol Biochem Behav. 2021;201:173093.
Golden SA, Covington HE, Berton O, Russo SJ. A standardized protocol for repeated social defeat stress in mice. Nat Protoc. 2011;6:1183–91.
Shen C-J, Zheng D, Li K-X, Yang J-M, Pan H-Q, Yu X-D, et al. Cannabinoid CB1 receptors in the amygdalar cholecystokinin glutamatergic afferents to nucleus accumbens modulate depressive-like behavior. Nat Med. 2019;25:337–49.
Chaudhury D, Walsh JJ, Friedman AK, Juarez B, Ku SM, Koo JW, et al. Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature. 2013;493:532–6.
Pittenger C, Duman RS. Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology. 2008;33:88–109.
Appelbaum LG, Shenasa MA, Stolz L, Daskalakis Z. Synaptic plasticity and mental health: methods, challenges and opportunities. Neuropsychopharmacology. 2023;48:113–20.
Roozendaal B, McEwen BS, Chattarji S. Stress, memory and the amygdala. Nat Rev Neurosci. 2009;10:423–33.
Cahill L, Babinsky R, Markowitsch HJ, McGaugh JL. The amygdala and emotional memory. Nature. 1995;377:295–6.
Nabavi S, Fox R, Proulx CD, Lin JY, Tsien RY, Malinow R. Engineering a memory with LTD and LTP. Nature. 2014;511:348–52.
Collingridge GL, Peineau S, Howland JG, Wang YT. Long-term depression in the CNS. Nat Rev Neurosci. 2010;11:459–73.
Wang H, Qian T, Zhao Y, Zhuo Y, Wu C, Osakada T, et al. A toolkit of highly selective and sensitive genetically encoded neuropeptide sensors. bioRxiv. https://doi.org/10.1101/2022.03.26.4859.
Li H, Namburi P, Olson JM, Borio M, Lemieux ME, Beyeler A, et al. Neurotensin orchestrates valence assignment in the amygdala. Nature. 2022;608:586–92.
Schneider F, Grodd W, Weiss U, Klose U, Mayer KR, Nägele T, et al. Functional MRI reveals left amygdala activation during emotion. Psychiatry Res. 1997;76:75–82.
Rodgers R, Cao B-J, Dalvi A, Holmes A. Animal models of anxiety: an ethological perspective. Braz J Med Biol Res. 1997;30:289–304.
Gray JA. Précis of The neuropsychology of anxiety: An enquiry into the functions of the septo-hippocampal system. Behav Brain Sci. 1982;5:469–84.
Lister RG. The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology. 1987;92:180–5.
Kalueff AV, Jensen CL, Murphy DL. Locomotory patterns, spatiotemporal organization of exploration and spatial memory in serotonin transporter knockout mice. Brain Res. 2007;1169:87–97.
Denenberg VH. Open‐field behavior in the rat: What does it mean? Ann N. Y Acad Sci. 1969;159:852–9.
Stanford SC. The open field test: reinventing the wheel. J Psychopharmacol. 2007;21:134–6.
Nishida A, Miyata K, Tsutsumi R, Yuki H, Akuzawa S, Kobayashi A, et al. Pharmacological profile of (R)-1-[2, 3-dihydro-1-(2’-methylphenacyl)-2-oxo-5-phenyl-1H-1, 4-benzodiazepin-3-yl]-3-(3-methylphenyl) urea (YM022), a new potent and selective gastrin/cholecystokinin-B receptor antagonist, in vitro and in vivo. J Pharmacol Exp Therap. 1994;269:725–31.
Semple G, Ryder H, Rooker DP, Batt AR, Kendrick DA, Szelke M, et al. (3 R)-N-(1-(tert-Butylcarbonylmethyl)-2, 3-dihydro-2-oxo-5-(2-pyridyl)-1 H-1, 4-benzodiazepin-3-yl)-N ‘-(3-(methylamino) phenyl) urea (YF476): A Potent and Orally Active Gastrin/CCK-B Antagonist. J Med Chem. 1997;40:331–41.
Pardridge WM. The blood-brain barrier: bottleneck in brain drug development. NeuroRx. 2005;2:3–14.
Cryan JF, Mombereau C, Vassout A. The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice. Neurosci Biobehav Rev. 2005;29:571–625.
Castagné V, Moser P, Roux S, Porsolt RD. Rodent models of depression: forced swim and tail suspension behavioral despair tests in rats and mice. Curr Protoc Pharmacol. 2010;49:5.8. 1-5.8. 14.
Jahangard L, Solgy R, Salehi I, Taheri SK, Holsboer-Trachsler E, Haghighi M, et al. Cholecystokinin (CCK) level is higher among first time suicide attempters than healthy controls, but is not associated with higher depression scores. Psychiatry Res. 2018;266:40–46.
Czéh B, Vardya I, Varga Z, Febbraro F, Csabai D, Martis L-S, et al. Long-term stress disrupts the structural and functional integrity of GABAergic neuronal networks in the medial prefrontal cortex of rats. Front Cell Neurosci. 2018;12:148.
Humeau Y, Choquet D. The next generation of approaches to investigate the link between synaptic plasticity and learning. Nat Neurosci. 2019;22:1536–43.
Josselyn SA, Tonegawa S. Memory engrams: Recalling the past and imagining the future. Science. 2020;367:eaaw4325.
Wu C-H, Ramos R, Katz DB, Turrigiano GG. Homeostatic synaptic scaling establishes the specificity of an associative memory. Curr Biol. 2021;31:2274–85.e2275.
Zheng Z, Guo C, Li M, Yang L, Liu P, Zhang X, et al. Hypothalamus-habenula potentiation encodes chronic stress experience and drives depression onset. Neuron. 2022;110:1400–15. e1406
Duman RS, Aghajanian GK, Sanacora G, Krystal JH. Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat Med. 2016;22:238–49.
Hamilton JP, Siemer M, Gotlib IH. Amygdala volume in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Mol Psychiatry. 2008;13:993–1000.
Drevets WC, Bogers W, Raichle ME. Functional anatomical correlates of antidepressant drug treatment assessed using PET measures of regional glucose metabolism. Eur Neuropsychopharmacol. 2002;12:527–44.
Beinfeld MC, Meyer DK, Eskay RL, Jensen RT, Brownstein MJ. The distribution of cholecystokinin immunoreactivity in the central nervous system of the rat as determined by radioimmunoassay. Brain Res. 1981;212:51–57.
Rehfeld JF. Immunochemical studies on cholecystokinin. II. Distribution and molecular heterogeneity in the central nervous system and small intestine of man and hog. J Biol Chem. 1978;253:4022–30.
Bullitt E. Expression of c‐fos‐like protein as a marker for neuronal activity following noxious stimulation in the rat. J Comp Neurol. 1990;296:517–30.
Kovács K. Measurement of immediate‐early gene activation‐c‐fos and beyond. J Neuroendocrinol. 2008;20:665–72.
Groenewold NA, Opmeer EM, de Jonge P, Aleman A, Costafreda SG. Emotional valence modulates brain functional abnormalities in depression: evidence from a meta-analysis of fMRI studies. Neurosci Biobehav Rev. 2013;37:152–63.
Hamilton JP, Etkin A, Furman DJ, Lemus MG, Johnson RF, Gotlib IH. Functional neuroimaging of major depressive disorder: a meta-analysis and new integration of baseline activation and neural response data. Am J Psychiatry. 2012;169:693–703.
Bradwejn J, Koszycki D, Paradis M, Reece P, Hinton J, Sedman A. Effect of CI-988 on cholecystokinin tetrapeptide-induced panic symptoms in healthy volunteers. Biol Psychiatry. 1995;38:742–6.
Derkach VA, Oh MC, Guire ES, Soderling TR. Regulatory mechanisms of AMPA receptors in synaptic plasticity. Nat Rev Neurosci. 2007;8:101–13.
Diering GH, Huganir RL. The AMPA receptor code of synaptic plasticity. Neuron. 2018;100:314–29.
Yi E-S, Oh S, Lee J-K, Leem Y-H. Chronic stress-induced dendritic reorganization and abundance of synaptosomal PKA-dependent CP-AMPA receptor in the basolateral amygdala in a mouse model of depression. Biochem Biophys Res Commun. 2017;486:671–8.
Zhou H-Y, He J-G, Hu Z-L, Xue S-G, Xu J-F, Cui Q-Q, et al. A-kinase anchoring protein 150 and protein kinase A complex in the basolateral amygdala contributes to depressive-like behaviors induced by chronic restraint stress. Biol Psychiatry. 2019;86:131–42.
Gibbons AS, Brooks L, Scarr E, Dean B. AMPA receptor expression is increased post-mortem samples of the anterior cingulate from subjects with major depressive disorder. J Affect Disord. 2012;136:1232–7.
Zanos P, Gould T. Mechanisms of ketamine action as an antidepressant. Mol Psychiatry. 2018;23:801–11.
Liu R-J, Lee FS, Li X-Y, Bambico F, Duman RS, Aghajanian GK. Brain-derived neurotrophic factor Val66Met allele impairs basal and ketamine-stimulated synaptogenesis in prefrontal cortex. Biol Psychiatry. 2012;71:996–1005.
Holz A, Mülsch F, Schwarz MK, Hollmann M, Döbrössy MD, Coenen VA, et al. Enhanced mGlu5 signaling in excitatory neurons promotes rapid antidepressant effects via AMPA receptor activation. Neuron. 2019;104:338–52.e337.
Carmona MA, Martínez A, Soler A, Blasi J, Soriano E, Aguado F. Ca2+-evoked synaptic transmission and neurotransmitter receptor levels are impaired in the forebrain of trkb (−/−) mice. Mol Cell Neurosci. 2003;22:210–26.
Acknowledgements
This work was supported by: Hong Kong Research Grants Council, General Research Fund: 11103220, 11101521, 11102422; (GRF, JFH) Hong Kong Research Grants Council, Collaborative Research Fund: C1043-21GF; (CRF, JFH) Innovation and Technology Fund: MRP/053/18X, GHP_075_19GD; (ITF, JFH) Health and Medical Research Fund: 06172456, 09203656; (HMRF, XC, JFH) and the following charitable foundations for their generous support to JFH: Wong Chun Hong Endowed Chair Professorship, Charlie Lee Charitable Foundation, and Fong Shu Fook Tong Foundation.
Author information
Authors and Affiliations
Contributions
JH, XZ, and MA designed the experiments; XZ, QG, and SW carried out the LTP study. XZ and MA performed the behavior experiments. MA and WF did the viral injections and cannula implantation surgeries. MA conducted optogenetic and fiber photometry recording experiments. WF, YL, KK, and HF performed histology experiments. MA and HW collected and analyzed the data for the western blotting. HM carried out the BBB penetration experiment. XZ and MA wrote the manuscript. JH assisted in editing and writing the manuscript. JH supervised the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, X., Asim, M., Fang, W. et al. Cholecystokinin B receptor antagonists for the treatment of depression via blocking long-term potentiation in the basolateral amygdala. Mol Psychiatry 28, 3459–3474 (2023). https://doi.org/10.1038/s41380-023-02127-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-023-02127-7
This article is cited by
-
Roles of KCNA2 in Neurological Diseases: from Physiology to Pathology
Molecular Neurobiology (2024)