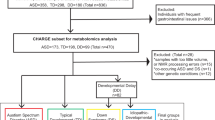
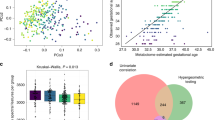
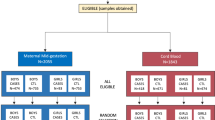
Abstract
The discovery of prenatal and neonatal molecular biomarkers has the potential to yield insights into autism spectrum disorder (ASD) and facilitate early diagnosis. We characterized metabolomic profiles in ASD using plasma samples collected in the Norwegian Autism Birth Cohort from mothers at weeks 17–21 gestation (maternal mid-gestation, MMG, n = 408) and from children on the day of birth (cord blood, CB, n = 418). We analyzed associations using sex-stratified adjusted logistic regression models with Bayesian analyses. Chemical enrichment analyses (ChemRICH) were performed to determine altered chemical clusters. We also employed machine learning algorithms to assess the utility of metabolomics as ASD biomarkers. We identified ASD associations with a variety of chemical compounds including arachidonic acid, glutamate, and glutamine, and metabolite clusters including hydroxy eicospentaenoic acids, phosphatidylcholines, and ceramides in MMG and CB plasma that are consistent with inflammation, disruption of membrane integrity, and impaired neurotransmission and neurotoxicity. Girls with ASD have disruption of ether/non-ether phospholipid balance in the MMG plasma that is similar to that found in other neurodevelopmental disorders. ASD boys in the CB analyses had the highest number of dysregulated chemical clusters. Machine learning classifiers distinguished ASD cases from controls with area under the receiver operating characteristic (AUROC) values ranging from 0.710 to 0.853. Predictive performance was better in CB analyses than in MMG. These findings may provide new insights into the sex-specific differences in ASD and have implications for discovery of biomarkers that may enable early detection and intervention.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Maenner MJ, Shaw KA, Bakian AV, Bilder DA, Durkin MS, Esler A, et al. Prevalence and characteristics of autism spectrum disorder among children aged 8 years - Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2018. MMWR Surveill Summ. 2021;70:1–16.
Towle PO, Patrick PA, Ridgard T, Pham S, Marrus J. Is earlier better? The relationship between age when starting early intervention and outcomes for children with autism spectrum disorder: a selective review. Autism Res Treat. 2020;2020:7605876.
Wallace KS, Rogers SJ. Intervening in infancy: implications for autism spectrum disorders. J Child Psychol Psychiatry. 2010;51:1300–20.
Zwaigenbaum L, Penner M. Autism spectrum disorder: advances in diagnosis and evaluation. BMJ 2018;361:k1674.
Siu MT, Weksberg R. Epigenetics of autism spectrum disorder. Adv Exp Med Biol. 2017;978:63–90.
Ritz B, Yan Q, Uppal K, Liew Z, Cui X, Ling C, et al. Untargeted metabolomics screen of mid-pregnancy maternal serum and autism in offspring. Autism Res. 2020;13:1258–69.
Schmidt RJ, Liang D, Busgang SA, Curtin P, Giulivi C. Maternal plasma metabolic profile demarcates a role for neuroinflammation in non-typical development of children. Metabolites. 2021;11:545.
Panjwani AA, Ji Y, Fahey JW, Palmer A, Wang G, Hong X, et al. Maternal dyslipidemia, plasma branched-chain amino acids, and the risk of child autism spectrum disorder: evidence of sex difference. J Autism Dev Disord. 2020;50:540–50.
Courraud J, Ernst M, Svane Laursen S, Hougaard DM, Cohen AS. Studying autism using untargeted metabolomics in newborn screening samples. J Mol Neurosci. 2021;71:1378–93.
Barone R, Alaimo S, Messina M, Pulvirenti A, Bastin J, Group MI-A, et al. A subset of patients with autism spectrum disorders show a distinctive metabolic profile by dried blood spot analyses. Front Psychiatry. 2018;9:636.
Stoltenberg C, Schjolberg S, Bresnahan M, Hornig M, Hirtz D, Dahl C, et al. The Autism Birth Cohort: a paradigm for gene-environment-timing research. Mol Psychiatry. 2010;15:676–80.
Magnus P, Irgens LM, Haug K, Nystad W, Skjaerven R, Stoltenberg C, et al. Cohort profile: the Norwegian Mother and Child Cohort Study (MoBa). Int J Epidemiol. 2006;35:1146–50.
Magnus P, Birke C, Vejrup K, Haugan A, Alsaker E, Daltveit AK, et al. Cohort profile update: The Norwegian Mother and Child Cohort Study (MoBa). Int J Epidemiol. 2016;45:382–8.
Bresnahan M, Hornig M, Schultz AF, Gunnes N, Hirtz D, Lie KK, et al. Association of maternal report of infant and toddler gastrointestinal symptoms with autism: evidence from a prospective birth cohort. JAMA Psychiatry. 2015;72:466–74.
Association AP. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR). American Psychiatric Association: Arlington. 2000.
Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24:659–85.
Lord C, Risi S, Lambrecht L, Cook EH Jr., Leventhal BL, DiLavore PC, et al. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30:205–23.
Organization WH. International classification of diseases and related health problems, 10th revision. Geneva: World Health Organization. 1992.
Strand BH, Dalgard OS, Tambs K, Rognerud M. Measuring the mental health status of the Norwegian population: a comparison of the instruments SCL-25, SCL-10, SCL-5 and MHI-5 (SF-36). Nord J Psychiatry. 2003;57:113–8.
Ronningen KS, Paltiel L, Meltzer HM, Nordhagen R, Lie KK, Hovengen R, et al. The biobank of the Norwegian Mother and Child Cohort Study: a resource for the next 100 years. Eur J Epidemiol. 2006;21:619–25.
Fiehn O. Metabolomics by gas chromatography-mass spectrometry: combined targeted and untargeted profiling. Curr Protoc Mol Biol. 2016;114:30 4 1–4 2.
Kind T, Wohlgemuth G, Lee DY, Lu Y, Palazoglu M, Shahbaz S, et al. FiehnLib: mass spectral and retention index libraries for metabolomics based on quadrupole and time-of-flight gas chromatography/mass spectrometry. Anal Chem. 2009;81:10038–48.
Cajka T, Smilowitz JT, Fiehn O. Validating quantitative untargeted lipidomics across nine liquid chromatography-high-resolution mass spectrometry platforms. Anal Chem. 2017;89:12360–8.
Tsugawa H, Cajka T, Kind T, Ma Y, Higgins B, Ikeda K, et al. MS-DIAL: data-independent MS/MS deconvolution for comprehensive metabolome analysis. Nat Methods. 2015;12:523–6.
Tsugawa H, Ikeda K, Takahashi M, Satoh A, Mori Y, Uchino H, et al. A lipidome atlas in MS-DIAL 4. Nat Biotechnol. 2020;38:1159–63.
Wohlgemuth G, Mehta SS, Mejia RF, Neumann S, Pedrosa D, Pluskal T, et al. SPLASH, a hashed identifier for mass spectra. Nat Biotechnol. 2016;34:1099–101.
DeFelice BC, Mehta SS, Samra S, Cajka T, Wancewicz B, Fahrmann JF, et al. Mass Spectral Feature List Optimizer (MS-FLO): A tool to minimize false positive peak reports in untargeted Liquid Chromatography-Mass Spectroscopy (LC-MS) Data Processing. Anal Chem. 2017;89:3250–5.
Fan S, Kind T, Cajka T, Hazen SL, Tang WHW, Kaddurah-Daouk R, et al. Systematic error removal using random forest for normalizing large-scale untargeted lipidomics data. Anal Chem. 2019;91:3590–6.
Barupal DK, Fiehn O. Chemical Similarity Enrichment Analysis (ChemRICH) as alternative to biochemical pathway mapping for metabolomic datasets. Sci Rep. 2017;7:14567.
Brydges C, Che X, Lipkin WI, Fiehn O. Bayesian statistics improves biological interpretability of metabolomics data from human cohorts. bioRxiv. 2022. https://doi.org/10.1101/2022.05.17.492312.
Jeffreys H. Theory of Probability. 3rd ed. Oxford: Clarendon Press; 1961.
Tibshirani R. Regression shrinkage and selection via the Lasso. J R Stat Soc: Ser B (Methodol). 1996;58:267–88.
Zou H. The adaptive Lasso and its Oracle properties. J Am Stat Assoc. 2012;101:1418–29.
Breiman L. Random forests. Mach Learn. 2001;45:5–32.
Chen T, Guestrin C. XGBoost: A Scalable Tree Boosting System. 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining; August 13–17, 2016; San Francisco, CA2016. 785-94.
Candes E, Fan Y, Janson L, Lv J. Panning for gold:‘model‐X’knockoffs for high dimensional controlled variable selection. J R Stat Soc: Ser B (Stat Methodol). 2018;80:551–77.
Johnson WE, Li C, Rabinovic A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics 2007;8:118–27.
Cappelletti M, Della Bella S, Ferrazzi E, Mavilio D, Divanovic S. Inflammation and preterm birth. J Leukoc Biol. 2016;99:67–78.
Parletta N, Niyonsenga T, Duff J. Omega-3 and Omega-6 polyunsaturated fatty acid levels and correlations with symptoms in children with attention deficit hyperactivity disorder, autistic spectrum disorder and typically developing controls. PLoS One. 2016;11:e0156432.
Al-Otaish H, Al-Ayadhi L, Bjorklund G, Chirumbolo S, Urbina MA, El-Ansary A. Relationship between absolute and relative ratios of glutamate, glutamine and GABA and severity of autism spectrum disorder. Metab Brain Dis. 2018;33:843–54.
El-Ansary A. Data of multiple regressions analysis between selected biomarkers related to glutamate excitotoxicity and oxidative stress in Saudi autistic patients. Data Brief. 2016;7:111–6.
Van Meter KC, Christiansen LE, Delwiche LD, Azari R, Carpenter TE, Hertz-Picciotto I. Geographic distribution of autism in California: a retrospective birth cohort analysis. Autism Res. 2010;3:19–29.
Suren P, Roth C, Bresnahan M, Haugen M, Hornig M, Hirtz D, et al. Association between maternal use of folic acid supplements and risk of autism spectrum disorders in children. JAMA 2013;309:570–7.
Wiggs KK, Rickert ME, Sujan AC, Quinn PD, Larsson H, Lichtenstein P, et al. Antiseizure medication use during pregnancy and risk of ASD and ADHD in children. Neurology 2020;95:e3232–e40.
Stromland K, Nordin V, Miller M, Akerstrom B, Gillberg C. Autism in thalidomide embryopathy: a population study. Dev Med Child Neurol. 1994;36:351–6.
Smith SE, Li J, Garbett K, Mirnics K, Patterson PH. Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci. 2007;27:10695–702.
Nicolini C, Fahnestock M. The valproic acid-induced rodent model of autism. Exp Neurol. 2018;299:217–27.
Patterson PH. Maternal infection and immune involvement in autism. Trends Mol Med. 2011;17:389–94.
Parenti M, Schmidt RJ, Ozonoff S, Shin HM, Tancredi DJ, Krakowiak P, et al. Maternal serum and placental metabolomes in association with prenatal phthalate exposure and neurodevelopmental outcomes in the MARBLES Cohort. Metabolites. 2022;12:829.
Hollowood K, Melnyk S, Pavliv O, Evans T, Sides A, Schmidt RJ, et al. Maternal metabolic profile predicts high or low risk of an autism pregnancy outcome. Res Autism Spectr Disord. 2018;56:72–82.
Girchenko P, Lahti-Pulkkinen M, Lipsanen J, Heinonen K, Lahti J, Rantalainen V, et al. Maternal early-pregnancy body mass index-associated metabolomic component and mental and behavioral disorders in children. Mol Psychiatry. 2022;27:4653–61.
Lyall K, Windham GC, Snyder NW, Kuskovsky R, Xu P, Bostwick A, et al. Association between midpregnancy polyunsaturated fatty acid levels and offspring autism spectrum disorder in a California population-based case-control study. Am J Epidemiol. 2021;190:265–76.
Kim JH, Yan Q, Uppal K, Cui X, Ling C, Walker DI, et al. Metabolomics analysis of maternal serum exposed to high air pollution during pregnancy and risk of autism spectrum disorder in offspring. Environ Res. 2021;196:110823.
Ferdinandusse S, McWalter K, Te Brinke H, L IJ, Mooijer PM, Ruiter JPN, et al. An autosomal dominant neurological disorder caused by de novo variants in FAR1 resulting in uncontrolled synthesis of ether lipids. Genet Med. 2021;23:740–50.
Staps P, Rizzo WB, Vaz FM, Bugiani M, Giera M, Heijs B, et al. Disturbed brain ether lipid metabolism and histology in Sjogren-Larsson syndrome. J Inherit Metab Dis. 2020;43:1265–78.
Vaz FM, McDermott JH, Alders M, Wortmann SB, Kolker S, Pras-Raves ML, et al. Mutations in PCYT2 disrupt etherlipid biosynthesis and cause a complex hereditary spastic paraplegia. Brain 2019;142:3382–97.
Teruya T, Chen YJ, Kondoh H, Fukuji Y, Yanagida M. Whole-blood metabolomics of dementia patients reveal classes of disease-linked metabolites. Proc Natl Acad Sci USA. 2021;118:e2022857118.
Bjornevik K, Zhang Z, O’Reilly EJ, Berry JD, Clish CB, Deik A, et al. Prediagnostic plasma metabolomics and the risk of amyotrophic lateral sclerosis. Neurology 2019;92:e2089–e100.
Yang F, Wu SC, Ling ZX, Chao S, Zhang LJ, Yan XM, et al. Altered plasma metabolic profiles in chinese patients with multiple sclerosis. Front Immunol. 2021;12:792711.
Liao X, Yang J, Wang H, Li Y. Microglia mediated neuroinflammation in autism spectrum disorder. J Psychiatr Res. 2020;130:167–76.
Gupta S, Ellis SE, Ashar FN, Moes A, Bader JS, Zhan J, et al. Transcriptome analysis reveals dysregulation of innate immune response genes and neuronal activity-dependent genes in autism. Nat Commun. 2014;5:5748.
Balic A, Vlasic D, Zuzul K, Marinovic B, Bukvic Mokos Z. Omega-3 Versus Omega-6 polyunsaturated fatty acids in the prevention and treatment of inflammatory skin diseases. Int J Mol Sci. 2020;21:741.
So J, Wu D, Lichtenstein AH, Tai AK, Matthan NR, Maddipati KR, et al. EPA and DHA differentially modulate monocyte inflammatory response in subjects with chronic inflammation in part via plasma specialized pro-resolving lipid mediators: A randomized, double-blind, crossover study. Atherosclerosis 2021;316:90–8.
Onodera T, Fukuhara A, Shin J, Hayakawa T, Otsuki M, Shimomura I. Eicosapentaenoic acid and 5-HEPE enhance macrophage-mediated Treg induction in mice. Sci Rep. 2017;7:4560.
Kishikawa A, Kitaura H, Kimura K, Ogawa S, Qi J, Shen WR, et al. Docosahexaenoic acid inhibits inflammation-induced Osteoclast formation and bone resorption in vivo through GPR120 by inhibiting TNF-alpha production in macrophages and directly inhibiting osteoclast formation. Front Endocrinol. 2019;10:157.
Kikut J, Komorniak N, Zietek M, Palma J, Szczuko M. Inflammation with the participation of arachidonic (AA) and linoleic acid (LA) derivatives (HETEs and HODEs) is necessary in the course of a normal reproductive cycle and pregnancy. J Reprod Immunol. 2020;141:103177.
Rizzo MT, Carlo-Stella C. Arachidonic acid mediates interleukin-1 and tumor necrosis factor-alpha-induced activation of the c-jun amino-terminal kinases in stromal cells. Blood 1996;88:3792–800.
Hughes-Fulford M, Li CF, Boonyaratanakornkit J, Sayyah S. Arachidonic acid activates phosphatidylinositol 3-kinase signaling and induces gene expression in prostate cancer. Cancer Res. 2006;66:1427–33.
Node K, Huo Y, Ruan X, Yang B, Spiecker M, Ley K, et al. Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science 1999;285:1276–9.
Ghazali R, Mehta KJ, Bligh SA, Tewfik I, Clemens D, Patel VB. High omega arachidonic acid/docosahexaenoic acid ratio induces mitochondrial dysfunction and altered lipid metabolism in human hepatoma cells. World J Hepatol. 2020;12:84–98.
Cotogni P, Muzio G, Trombetta A, Ranieri VM, Canuto RA. Impact of the omega-3 to omega-6 polyunsaturated fatty acid ratio on cytokine release in human alveolar cells. JPEN J Parenter Enter Nutr. 2011;35:114–21.
Che X, Hornig M, Bresnahan M, Stoltenberg C, Magnus P, Suren P, et al. Maternal mid-gestational and child cord blood immune signatures are strongly associated with offspring risk of ASD. Mol Psychiatry. 2022;27:1527–41.
Dawaliby R, Trubbia C, Delporte C, Masureel M, Van Antwerpen P, Kobilka BK, et al. Allosteric regulation of G protein-coupled receptor activity by phospholipids. Nat Chem Biol. 2016;12:35–9.
van der Veen JN, Kennelly JP, Wan S, Vance JE, Vance DE, Jacobs RL. The critical role of phosphatidylcholine and phosphatidylethanolamine metabolism in health and disease. Biochim Biophys Acta Biomembr. 2017;1859:1558–72. (9 Pt B)
Schuler MH, Di Bartolomeo F, Bottinger L, Horvath SE, Wenz LS, Daum G, et al. Phosphatidylcholine affects the role of the sorting and assembly machinery in the biogenesis of mitochondrial beta-barrel proteins. J Biol Chem. 2015;290:26523–32.
Niebergall LJ, Vance DE. The ratio of phosphatidylcholine to phosphatidylethanolamine does not predict integrity of growing MT58 Chinese hamster ovary cells. Biochim Biophys Acta. 2012;1821:324–34.
Kano-Sueoka T, Nicks ME. Abnormal function of protein kinase C in cells having phosphatidylethanolamine-deficient and phosphatidylcholine-excess membranes. Cell Growth Differ. 1993;4:533–7.
Rockenfeller P, Koska M, Pietrocola F, Minois N, Knittelfelder O, Sica V, et al. Phosphatidylethanolamine positively regulates autophagy and longevity. Cell Death Differ. 2015;22:499–508.
Longo N, Frigeni M, Pasquali M. Carnitine transport and fatty acid oxidation. Biochim Biophys Acta. 2016;1863:2422–35.
Needham BD, Adame MD, Serena G, Rose DR, Preston GM, Conrad MC, et al. Plasma and fecal metabolite profiles in autism spectrum disorder. Biol Psychiatry. 2021;89:451–62.
Thomas RH, Foley KA, Mepham JR, Tichenoff LJ, Possmayer F, MacFabe DF. Altered brain phospholipid and acylcarnitine profiles in propionic acid infused rodents: further development of a potential model of autism spectrum disorders. J Neurochem. 2010;113:515–29.
Uche LE, Gooris GS, Bouwstra JA, Beddoes CM. Increased levels of short-chain ceramides modify the lipid organization and reduce the lipid barrier of skin model membranes. Langmuir 2021;37:9478–89.
Hussain MM, Jin W, Jiang XC. Mechanisms involved in cellular ceramide homeostasis. Nutr Metab. 2012;9:71.
Melland-Smith M, Ermini L, Chauvin S, Craig-Barnes H, Tagliaferro A, Todros T, et al. Disruption of sphingolipid metabolism augments ceramide-induced autophagy in preeclampsia. Autophagy 2015;11:653–69.
Ross MM, Piorczynski TB, Harvey J, Burnham TS, Francis M, Larsen MW, et al. Ceramide: a novel inducer for neural tube defects. Dev Dyn. 2019;248:979–96.
Ariga T, Jarvis WD, Yu RK. Role of sphingolipid-mediated cell death in neurodegenerative diseases. J Lipid Res. 1998;39:1–16.
Stoica BA, Movsesyan VA, Lea PMt, Faden AI. Ceramide-induced neuronal apoptosis is associated with dephosphorylation of Akt, BAD, FKHR, GSK-3beta, and induction of the mitochondrial-dependent intrinsic caspase pathway. Mol Cell Neurosci. 2003;22:365–82.
France-Lanord V, Brugg B, Michel PP, Agid Y, Ruberg M. Mitochondrial free radical signal in ceramide-dependent apoptosis: a putative mechanism for neuronal death in Parkinson’s disease. J Neurochem. 1997;69:1612–21.
Fanani ML, Maggio B. The many faces (and phases) of ceramide and sphingomyelin I - single lipids. Biophys Rev. 2017;9:589–600.
Rogasevskaia T, Coorssen JR. Sphingomyelin-enriched microdomains define the efficiency of native Ca(2+)-triggered membrane fusion. J Cell Sci. 2006;11913:2688–94.
Corcelle-Termeau E, Vindelov SD, Hamalisto S, Mograbi B, Keldsbo A, Brasen JH, et al. Excess sphingomyelin disturbs ATG9A trafficking and autophagosome closure. Autophagy 2016;12:833–49.
MahmoudianDehkordi S, Ahmed AT, Bhattacharyya S, Han X, Baillie RA, Arnold M, et al. Alterations in acylcarnitines, amines, and lipids inform about the mechanism of action of citalopram/escitalopram in major depression. Transl Psychiatry. 2021;11:153.
Bhattacharyya S, Ahmed AT, Arnold M, Liu D, Luo C, Zhu H, et al. Metabolomic signature of exposure and response to citalopram/escitalopram in depressed outpatients. Transl Psychiatry. 2019;9:173.
Dorninger F, Forss-Petter S, Berger J. From peroxisomal disorders to common neurodegenerative diseases - the role of ether phospholipids in the nervous system. FEBS Lett. 2017;591:2761–88.
Frazier TW, Georgiades S, Bishop SL, Hardan AY. Behavioral and cognitive characteristics of females and males with autism in the Simons Simplex Collection. J Am Acad Child Adolesc Psychiatry. 2014;53:329–40.e1.-3
Aldred S, Moore KM, Fitzgerald M, Waring RH. Plasma amino acid levels in children with autism and their families. J Autism Dev Disord. 2003;33:93–7.
Shimmura C, Suda S, Tsuchiya KJ, Hashimoto K, Ohno K, Matsuzaki H, et al. Alteration of plasma glutamate and glutamine levels in children with high-functioning autism. PLoS One. 2011;6:e25340.
Belov Kirdajova D, Kriska J, Tureckova J, Anderova M. Ischemia-triggered glutamate excitotoxicity from the perspective of Glial cells. Front Cell Neurosci. 2020;14:51.
Tseng EE, Brock MV, Lange MS, Troncoso JC, Blue ME, Lowenstein CJ, et al. Glutamate excitotoxicity mediates neuronal apoptosis after hypothermic circulatory arrest. Ann Thorac Surg. 2010;89:440–5.
Ankarcrona M, Dypbukt JM, Bonfoco E, Zhivotovsky B, Orrenius S, Lipton SA, et al. Glutamate-induced neuronal death: a succession of necrosis or apoptosis depending on mitochondrial function. Neuron 1995;15:961–73.
Takeuchi H, Jin S, Wang J, Zhang G, Kawanokuchi J, Kuno R, et al. Tumor necrosis factor-alpha induces neurotoxicity via glutamate release from hemichannels of activated microglia in an autocrine manner. J Biol Chem. 2006;281:21362–8.
Yang GY, Gong C, Qin Z, Liu XH, Lorris, Betz A. Tumor necrosis factor alpha expression produces increased blood-brain barrier permeability following temporary focal cerebral ischemia in mice. Brain Res Mol Brain Res. 1999;69:135–43.
Zhou Y, Danbolt NC. Glutamate as a neurotransmitter in the healthy brain. J Neural Transm. 2014;121:799–817.
Hassan TH, Abdelrahman HM, Fattah NRA, El-Masry NM, Hashim HM, El-Gerby KM, et al. Blood and brain glutamate levels in children with autistic disorder. Res Autism Spectr Disord. 2013;7:541–8.
Albrecht J, Sidoryk-Wegrzynowicz M, Zielinska M, Aschner M. Roles of glutamine in neurotransmission. Neuron Glia Biol. 2010;6:263–76.
Chen J, Herrup K. Glutamine acts as a neuroprotectant against DNA damage, beta-amyloid and H2O2-induced stress. PLoS One. 2012;7:e33177.
Stelmashook EV, Lozier ER, Goryacheva ES, Mergenthaler P, Novikova SV, Zorov DB, et al. Glutamine-mediated protection from neuronal cell death depends on mitochondrial activity. Neurosci Lett. 2010;482:151–5.
Budni J, Molz S, Dal-Cim T, Martin-de-Saavedra MD, Egea J, Lopez MG, et al. Folic acid protects against glutamate-induced excitotoxicity in hippocampal slices through a mechanism that implicates inhibition of GSK-3beta and iNOS. Mol Neurobiol. 2018;55:1580–9.
Acknowledgements
This paper is dedicated to the memory of Bohyun Lee and Pål Suren-two brilliant young investigators who contributed to the design, execution and analyses presented here. We thank Aaron Cheng, Ziqi Zhou, Yan Wang, and Yuenling Cheng for data preparation and management, James Ng for sample preparation and handling. We are grateful to the participants in the MoBa and ABC studies. This work was funded by National Institutes of Health grants NS047537 and NS086122, the Jane Botsford Johnson foundation, the Norwegian Ministry of Health and Care Services, the Norwegian Ministry of Education and Research, and Research Council of Norway grants 189457, 190694, and 196452. The funding agencies did not participate in study design, data collection and interpretation, or the decision to submit the work for publication.
Author information
Authors and Affiliations
Contributions
CS, ES, MB, PM, and WIL developed the experimental design. OF directed metabolomics assays. XC directed statistical analyses and data interpretations. AR, WIL, and XC wrote the manuscript. AR, CS, ES, KZ, MB, OF, PM, SM, TR-K, WIL, XC, and YS contributed to the data analyses, edited, and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Che, X., Roy, A., Bresnahan, M. et al. Metabolomic analysis of maternal mid-gestation plasma and cord blood in autism spectrum disorders. Mol Psychiatry 28, 2355–2369 (2023). https://doi.org/10.1038/s41380-023-02051-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-023-02051-w