Abstract
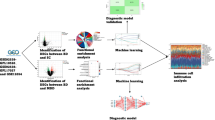
Psychosis onset is a transdiagnostic event that leads to a range of psychiatric disorders, which are currently diagnosed through clinical observation. The integration of multimodal biological data could reveal different subtypes of psychosis onset to target for the personalization of care. In this study, we tested the existence of subgroups of patients affected by first-episode psychosis (FEP) with a possible immunopathogenic basis. To do this, we designed a data-driven unsupervised machine learning model to cluster a sample of 127 FEP patients and 117 healthy controls (HC), based on the peripheral blood expression levels of 12 psychosis-related immune gene transcripts. To validate the model, we applied a resampling strategy based on the half-splitting of the total sample with random allocation of the cases. Further, we performed a post-hoc univariate analysis to verify the clinical, cognitive, and structural brain correlates of the subgroups identified. The model identified and validated two distinct clusters: 1) a FEP cluster characterized by the high expression of inflammatory and immune-activating genes (IL1B, CCR7, IL12A and CXCR3); 2) a cluster consisting of an equal number of FEP and HC subjects, which did not show a relative over or under expression of any immune marker (balanced subgroup). None of the subgroups was related to specific symptoms dimensions or longitudinal diagnosis of affective vs non-affective psychosis. FEP patients included in the balanced immune subgroup showed a thinning of the left supramarginal and superiorfrontal cortex (FDR-adjusted p-values < 0.05). Our results demonstrated the existence of a FEP patients’ subgroup identified by a multivariate pattern of immunomarkers involved in inflammatory activation. This evidence may pave the way to sample stratification in clinical studies aiming to develop diagnostic tools and therapies targeting specific immunopathogenic pathways of psychosis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The original data used in this study are available online as Supplementary information. Supplementary information is available at MP’s website.
Code availability
The original code of the machine learning model is publicly available in GitHub (https://htmlpreview.github.io/?https://github.com/UniMiPsychiatry/Immunopsychiatry/blob/main/Analyses.html).
References
Biedermann F, Fleischhacker WW. Psychotic disorders in DSM-5 and ICD-11. CNS Spectr. 2016;21:349–54.
Blacker D, Tsuang MT. Contested boundaries of bipolar disorder and the limits of categorical diagnosis in psychiatry. Am J Psychiatry. 1992;149:1473–83.
Fond G, Jamain S, Tamouza R, Arango C, Wolfgang Fleischhacker W, Glenthøj B, et al. The Promise of Biological Markers for Treatment Response in First-Episode Psychosis: A Systematic Review. Schizophr Bull. 2015;41:559–73.
Doherty JL, Owen MJ. Genomic insights into the overlap between psychiatric disorders: Implications for research and clinical practice. Genome Med. 2014;6:1–13.
Xia CH, Ma Z, Ciric R, Gu S, Betzel RF, Kaczkurkin AN, et al. Linked dimensions of psychopathology and connectivity in functional brain networks. Nat Commun. 2018;9:1. 2018;9:1–14
Voineskos AN, Jacobs GR, Ameis SH. Neuroimaging Heterogeneity in Psychosis: Neurobiological Underpinnings and Opportunities for Prognostic and Therapeutic Innovation. Biol Psychiatry. 2020;88:95.
Ethridge LE, Hamm JP, Pearlson GD, Tamminga CA, Sweeney JA, Keshavan MS, et al. Event-related potential and time-frequency endophenotypes for schizophrenia and psychotic bipolar disorder. Biol Psychiatry. 2015;77:127–36.
Kristian Hill S, Reilly JL, Keefe RSE, Gold JM, Bishop JR, Gershon ES, et al. Neuropsychological impairments in schizophrenia and psychotic bipolar disorder: findings from the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) study. Am J Psychiatry. 2013;170:1275–84.
Khadka S, Meda SA, Stevens MC, Glahn DC, Calhoun VD, Sweeney JA, et al. Is aberrant functional connectivity a psychosis endophenotype? A resting state functional magnetic resonance imaging study. Biol Psychiatry. 2013;74:458–66.
Reilly JL, Frankovich K, Hill S, Gershon ES, Keefe RSE, Keshavan MS, et al. Elevated Antisaccade Error Rate as an Intermediate Phenotype for Psychosis Across Diagnostic Categories. Schizophr Bull. 2014;40:1011.
Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167:748–51.
Feczko E, Miranda-Dominguez O, Marr M, Graham AM, Nigg JT, Fair DA. The Heterogeneity problem: Approaches to identify psychiatric subtypes. Trends Cogn Sci. 2019;23:584.
Insel TR, Cuthbert BN. Brain disorders? Precisely. Sci (1979) 2015;348:499–500.
Pigoni A, Dwyer D, Squarcina L, Borgwardt S, Crespo-Facorro B, Dazzan P, et al. Classification of first-episode psychosis using cortical thickness: A large multicenter MRI study. Eur Neuropsychopharmacol. 2021;47:34–47.
James G, Witten D, Hastie T, Tibshirani R. An Introduction to Statistical Learning. vol. 103. New York, NY: Springer New York; 2013.
Chand GB, Dwyer DB, Erus G, Sotiras A, Varol E, Srinivasan D, et al. Two distinct neuroanatomical subtypes of schizophrenia revealed using machine learning. Brain 2020;143:1027–38.
Martinuzzi E, Barbosa S, Daoudlarian D, Bel Haj Ali W, Gilet C, Fillatre L, et al. Stratification and prediction of remission in first-episode psychosis patients: the OPTiMiSE cohort study. Transl Psychiatry. 2019;9:1–20.
Stefanik L, Erdman L, Ameis SH, Foussias G, Mulsant BH, Behdinan T, et al. Brain-Behavior Participant Similarity Networks Among Youth and Emerging Adults with Schizophrenia Spectrum, Autism Spectrum, or Bipolar Disorder and Matched Controls. Neuropsychopharmacology. 2018;43:1180.
Wenzel J, Haas SS, Dwyer DB, Ruef A, Oeztuerk OF, Antonucci LA, et al. Cognitive subtypes in recent onset psychosis: distinct neurobiological fingerprints? Neuropsychopharmacology. 2021;46:1475–83.
Zheng W, Cai D, bin, Yang XH, Ungvari GS, Ng CH, Müller N, et al. Adjunctive celecoxib for schizophrenia: A meta-analysis of randomized, double-blind, placebo-controlled trials. J Psychiatr Res. 2017;92:139–46.
Xiang YQ, Zheng W, Wang S, bin, Yang XH, Cai D, bin, Ng CH, et al. Adjunctive minocycline for schizophrenia: A meta-analysis of randomized controlled trials. Eur Neuropsychopharmacol. 2017;27:8–18.
Girgis RR, Ciarleglio A, Choo T, Haynes G, Bathon JM, Cremers S, et al. A Randomized, Double-Blind, Placebo-Controlled Clinical Trial of Tocilizumab, An Interleukin-6 Receptor Antibody, For Residual Symptoms in Schizophrenia. Neuropsychopharmacology. 2018;43:6. 2017;43:1317–23
Pinto JV, Moulin TC, Amaral OB. On the transdiagnostic nature of peripheral biomarkers in major psychiatric disorders: A systematic review. Neurosci Biobehav Rev. 2017;83:97–108.
Lizano P, Lutz O, Xu Y, Rubin LH, Paskowitz L, Lee AM, et al. Multivariate relationships between peripheral inflammatory marker subtypes and cognitive and brain structural measures in psychosis. Mol Psychiatry. 2021;26:3430–43.
Hoang D, Xu Y, Lutz O, Bannai D, Zeng V, Bishop JR, et al. Inflammatory Subtypes in Antipsychotic-Naïve First-Episode Schizophrenia are Associated with Altered Brain Morphology and Topological Organization. Brain Behav Immun. 2022;100:297–308.
Enrico P, Delvecchio G, Turtulici N, Pigoni A, Villa FM, Perlini C, et al. Classification of Psychoses Based on Immunological Features: A Machine Learning Study in a Large Cohort of First-Episode and Chronic Patients. Schizophr Bull. 2021;47:1141–55.
Roomruangwong C, Noto C, Kanchanatawan B, Anderson G, Kubera M, Carvalho AF, et al. The Role of Aberrations in the Immune-Inflammatory Response System (IRS) and the Compensatory Immune-Regulatory Reflex System (CIRS) in Different Phenotypes of Schizophrenia: the IRS-CIRS Theory of Schizophrenia. Mol Neurobiol. 2020;57:778–97.
Noto MN, Maes M, Nunes SOV, Ota VK, Rossaneis AC, Verri WA, et al. Activation of the immune-inflammatory response system and the compensatory immune-regulatory system in antipsychotic naive first episode psychosis. Eur Neuropsychopharmacol. 2019;29:416–31.
Radhakrishnan R, Kaser M, Guloksuz S. The Link between the Immune System, Environment, and Psychosis. Schizophr Bull. 2017;43:693–7.
Ruggeri M, Bonetto C, Lasalvia A, de Girolamo G, Fioritti A, Rucci P, et al. A multi-element psychosocial intervention for early psychosis (GET UP PIANO TRIAL) conducted in a catchment area of 10 million inhabitants: study protocol for a pragmatic cluster randomized controlled trial. Trials. 2012;13:73.
Ruggeri M, Bonetto C, Lasalvia A, Fioritti A, de Girolamo G, Santonastaso P, et al. Feasibility and effectiveness of a multi-element psychosocial intervention for first-episode psychosis: Results from the cluster-randomized controlled GET UP PIANO trial in a catchment area of 10 million inhabitants. Schizophr Bull. 2015;41:1192–203.
Jablensky A, Sartorius N, Ernberg G, Anker M, Korten A, Cooper JE, et al. Schizophrenia: manifestations, incidence and course in different cultures A World Health Organization Ten-Country Study. Psychol Med Monogr Suppl. 1992;20:1–97.
Kübler U. Structured Clinical Interview for DSM-IV (SCID). In: Gellman MD, Turner JR, editors. Encyclopedia of Behavioral Medicine, New York, NY: Springer New York; 2013. p. 1919–20.
World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–4.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001;25:402–8.
Wing JK, Babor T, Brugha T, Burke J, Cooper JE, Giel R, et al. SCAN: Schedules fonr Clinical Assessment in Neuropsychiatry. Arch Gen Psychiatry. 1990;47:589–93.
World Health Organization. The ICD-10 classification of mental and behavioural disorders: clinical descriptions and diagnostic guidelines. Genève, Switzerland: World Health Organization; 1992.
Pillinger T, D’Ambrosio E, McCutcheon R, Howes OD. Is psychosis a multisystem disorder? A meta-review of central nervous system, immune, cardiometabolic, and endocrine alterations in first-episode psychosis and perspective on potential models. Mol Psychiatry. 2019;24:776–94.
Şahin ŞK, Şimsek L, Aksoy PG, Elboga G, Altindag A, Keskek ŞÖ. Relationship between the Visceral Adiposity Index and neutrophil/lymphocyte ratio in patients with bipolar disorder. Minerva 2021;62:5–11.
Tonna M, Ossola P, Marchesi C, Bettini E, Lasalvia A, Bonetto C, et al. Dimensional structure of first episode psychosis. Early Inter Psychiatry. 2019;13:1431–8.
Cabrera B, Bioque M, Penadés R, González-Pinto A, Parellada M, Bobes J, et al. Cognition and psychopathology in first-episode psychosis: are they related to inflammation? Psychol Med. 2016;46:2133–44.
Asarnow RF, MacCrimmon DJ. Attention/information processing, neuropsychological functioning, and thought disorder during the acute and partial recovery phases of schizophrenia: A longitudinal study. Psychiatry Res. 1982;7:309–19.
Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 2006;31:968–80.
Troyanskaya O, Cantor M, Sherlock G, Brown P, Hastie T, Tibshirani R, et al. Missing value estimation methods for DNA microarrays. Bioinformatics 2001;17:520–5.
Walther A, Nili H, Ejaz N, Alink A, Kriegeskorte N, Diedrichsen J. Reliability of dissimilarity measures for multi-voxel pattern analysis. Neuroimage 2016;137:188–200.
Charrad M, Ghazzali N, Boiteau V, Niknafs A. NbClust: An R Package for Determining the Relevant Number of Clusters in a Data Set. J Stat Softw. 2014;61:1–36.
Andreoletti G, Lanata CM, Trupin L, Paranjpe I, Jain TS, Nititham J, et al. Transcriptomic analysis of immune cells in a multi-ethnic cohort of systemic lupus erythematosus patients identifies ethnicity- and disease-specific expression signatures. Commun Biol. 2021;4:1–13.
Yehya N, Varisco BM, Thomas NJ, Wong HR, Christie JD, Feng R, et al. Peripheral blood transcriptomic sub-phenotypes of pediatric acute respiratory distress syndrome. Crit Care. 2020;24:1–10.
Luo C, Pi X, Hu N, Wang X, Xiao Y, Li S, et al. Subtypes of schizophrenia identified by multi-omic measures associated with dysregulated immune function. Mol Psychiatry. 2021;26:6926–36.
Clatworthy J, Buick D, Hankins M, Weinman J, Horne R. The use and reporting of cluster analysis in health psychology: A review. Br J Health Psychol. 2005;10:329–58.
Marquand AF, Wolfers T, Mennes M, Buitelaar J, Beckmann CF. Beyond Lumping and Splitting: A Review of Computational Approaches for Stratifying Psychiatric Disorders. Biol Psychiatry. 2016;1:433.
Narita A, Nagai M, Mizuno S, Ogishima S, Tamiya G, Ueki M, et al. Clustering by phenotype and genome-wide association study in autism. Transl Psychiatry. 2020;10:1–12.
Armstrong RA. When to use the Bonferroni correction. Ophthalmic Physiological Opt. 2014;34:502–8.
Smail MA, Wu X, Henkel ND, Eby HM, Herman JP, McCullumsmith RE, et al. Similarities and dissimilarities between psychiatric cluster disorders. Mol Psychiatry. 2021;26:9. 2021;26:4853–63.
Rand WM. Objective Criteria for the Evaluation of Clustering Methods. J Am Stat Assoc. 1971;66:846.
Kindler J, Lim CK, Weickert CS, Boerrigter D, Galletly C, Liu D, et al. Dysregulation of kynurenine metabolism is related to proinflammatory cytokines, attention, and prefrontal cortex volume in schizophrenia. Mol Psychiatry. 2020;25:2860–72.
Belikan P, Bühler U, Wolf C, Pramanik GK, Gollan R, Zipp F, et al. CCR7 on CD4 + T Cells Plays a Crucial Role in the Induction of Experimental Autoimmune Encephalomyelitis. J Immunol. 2018;200:2554–62.
Cosma NC, Üsekes B, Otto LR, Gerike S, Heuser I, Regen F, et al. M1/M2 polarization in major depressive disorder: Disentangling state from trait effects in an individualized cell-culture-based approach. Brain Behav Immun. 2021;94:185–95.
Schiweck C, Claes S, van Oudenhove L, Lafit G, Vaessen T, de Beeck GO, et al. Childhood trauma, suicide risk and inflammatory phenotypes of depression: insights from monocyte gene expression. Transl Psychiatry. 2020;10:1. 2020;10:1–12
Netea MG, Domínguez-Andrés J, Barreiro LB, Chavakis T, Divangahi M, Fuchs E, et al. Defining trained immunity and its role in health and disease. Nat Rev Immunol. 2020;20:6. 2020;20:375–88
Joosten SA, van Meijgaarden KE, Arend SM, Prins C, Oftung F, Korsvold GE, et al. Mycobacterial growth inhibition is associated with trained innate immunity. J Clin Invest. 2018;128:1837–51.
Hurlin PJ, Huang J. The MAX-interacting transcription factor network. Semin Cancer Biol. 2006;16:265–74.
Dezfouli S, Bakke A, Huang J, Wynshaw-Boris A, Hurlin PJ. Inflammatory disease and lymphomagenesis caused by deletion of the Myc antagonist Mnt in T cells. Mol Cell Biol. 2006;26:2080–92.
O’Donovan SM, Sullivan C, Koene R, Devine E, Hasselfeld K, Moody CL, et al. Cell-subtype-specific changes in adenosine pathways in schizophrenia. Neuropsychopharmacology 2018;43:1667–74.
Tarantino N, Leboyer M, Bouleau A, Hamdani N, Richard JR, Boukouaci W, et al. Natural killer cells in first-episode psychosis: an innate immune signature? Mol Psychiatry. 2021;26:5297–306.
Trovão N, Prata J, VonDoellinger O, Santos S, Barbosa M, Coelho R. Peripheral Biomarkers for First-Episode Psychosis—Opportunities from the Neuroinflammatory Hypothesis of Schizophrenia. Psychiatry Investig. 2019;16:177–84.
Boerrigter D, Weickert TW, Lenroot R, O’Donnell M, Galletly C, Liu D, et al. Using blood cytokine measures to define high inflammatory biotype of schizophrenia and schizoaffective disorder. J Neuroinflammation. 2017;14:188.
Fillman SG, Weickert TW, Lenroot RK, Catts SV, Bruggemann JM, Catts VS, et al. Elevated peripheral cytokines characterize a subgroup of people with schizophrenia displaying poor verbal fluency and reduced Broca’s area volume. Mol Psychiatry. 2016;21:1090–8.
MacDowell KS, García-Bueno B, Madrigal JL, Parellada M, Arango C, Micó JA, et al. Risperidone normalizes increased inflammatory parameters and restores anti-inflammatory pathways in a model of neuroinflammation. Int J Neuropsychopharmacol. 2013;16(Feb):121–35.
Martín-Hernández D, Bris ÁG, MacDowell KS, García-Bueno B, Madrigal JL, Leza JC, et al. Modulation of the antioxidant nuclear factor (erythroid 2-derived)-like 2 pathway by antidepressants in rats. Neuropharmacology 2016;103(Apr):79–91.
MacDowell KS, Munarriz-Cuezva E, Caso JR, Madrigal JL, Zabala A, Meana JJ, et al. Paliperidone reverts Toll-like receptor 3 signaling pathway activation and cognitive deficits in a maternal immune activation mouse model of schizophrenia. Neuropharmacology 2017;116(Apr):196–207.
Pollak TA, Lennox BR, Müller S, Benros ME, Prüss H, Tebartz van Elst L, et al. Autoimmune psychosis: an international consensus on an approach to the diagnosis and management of psychosis of suspected autoimmune origin. Lancet Psychiatry. 2020;7:93–108.
Sommer IE, van Westrhenen R, Begemann MJH, de Witte LD, Leucht S, Kahn RS. Efficacy of Anti-inflammatory Agents to Improve Symptoms in Patients With Schizophrenia: An Update. Schizophr Bull. 2014;40:181–91.
Çakici N, van Beveren NJM, Judge-Hundal G, Koola MM, Sommer IEC. An update on the efficacy of anti-inflammatory agents for patients with schizophrenia: a meta-analysis. Psychol Med. 2019;49:2307.
Kahn RS, Sommer IE. The neurobiology and treatment of first-episode schizophrenia. Mol Psychiatry. 2015;20:84–97.
Müller N, Myint AM, Krause D, Weidinger E, Schwarz MJ. Anti-inflammatory treatment in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2013;42:146–53.
Funding
This study was partially supported by the Italian Ministry of Health (GR-2019-12369479 to AP) and by the Fondazione Cariverona (Promoting research to improve quality of care; Sotto-obiettivo A9 “Disabilità cognitiva e comportamentale nelle demenze e nelle psicosi” to PB and MR). The study was also partially funded by ERANET NEURON JTC2018 “Mental Disorders” UNMET project (Neuron-051) to PB. The APC costs were funded by GrantRicercaCorrente, Italian Ministry of Health.
Author information
Authors and Affiliations
Consortia
Contributions
PB, PE and GD designed this particular study. MR, AL, MG, and PB designed the GET UP project. PB designed the First and Prevent projects and with MB the Mandrake project. PE and GD prepared the first version of the manuscript. NT carried out the data analysis. GIC, AF, and RF carried out the immune transcriptome analysis. MB, PB, CP, MGR, AP, AL, MB, MGR, LB-C, and MR coordinated subject recruitment and sample collection. MB, CB, MR, AL, and PB coordinated data management. CP, PS, AD, ST, MI, FB, and AV were involved in patient enrolment and assessment. All authors revised and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Enrico, P., Delvecchio, G., Turtulici, N. et al. A machine learning approach on whole blood immunomarkers to identify an inflammation-associated psychosis onset subgroup. Mol Psychiatry 28, 1190–1200 (2023). https://doi.org/10.1038/s41380-022-01911-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-022-01911-1
This article is cited by
-
Immunophenotypes in psychosis: is it a premature inflamm-aging disorder?
Molecular Psychiatry (2024)
-
Advances in Molecular Psychiatry – March 2023: mitochondrial function, stress, neuroinflammation – bipolar disorder, psychosis, and Alzheimer’s disease
Molecular Psychiatry (2023)