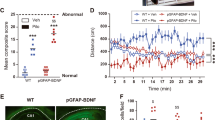
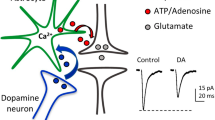
Abstract
The mechanisms underlying the dichotomic cortical/basal ganglia dopaminergic abnormalities in schizophrenia are unclear. Astrocytes are important non-neuronal modulators of brain circuits, but their role in dopaminergic system remains poorly explored. Microarray analyses, immunohistochemistry, and two-photon laser scanning microscopy revealed that Dys1 hypofunction increases the reactivity of astrocytes, which express only the Dys1A isoform. Notably, behavioral and electrochemical assessments in mice selectively lacking the Dys1A isoform unraveled a more prominent impact of Dys1A in behavioral and dopaminergic/D2 alterations related to basal ganglia, but not cortical functioning. Ex vivo electron microscopy and protein expression analyses indicated that selective Dys1A disruption might alter intracellular trafficking in astrocytes, but not in neurons. In agreement, Dys1A disruption only in astrocytes resulted in decreased motivation and sensorimotor gating deficits, increased astrocytic dopamine D2 receptors and decreased dopaminergic tone within basal ganglia. These processes might have clinical relevance because the caudate, but not the cortex, of patients with schizophrenia shows a reduction of the Dys1A isoform. Therefore, we started to show a hitherto unknown role for the Dys1A isoform in astrocytic-related modulation of basal ganglia behavioral and dopaminergic phenotypes, with relevance to schizophrenia.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Leggio GM, Torrisi SA, Mastrogiacomo R, Mauro D, Chisari M, Devroye C, et al. The epistatic interaction between the dopamine D3 receptor and dysbindin-1 modulates higher-order cognitive functions in mice and humans. Mol Psychiatry. 2021;26:1272–85. https://doi.org/10.1038/s41380-019-0511-4. Epub 2019 Sep 6.
Savage JE, Jansen PR, Stringer S, Watanabe K, Bryois J, de Leeuw CA, et al. Genome-wide association meta-analysis in 269,867 individuals identifies new genetic and functional links to intelligence. Nat Genet. 2018;50:912–9.
Scheggia D, Mastrogiacomo R, Mereu M, Sannino S, Straub RE, Armando M, et al. Variations in Dysbindin-1 are associated with cognitive response to antipsychotic drug treatment. Nat Commun. 2018;9:2265.
Waddington JL, Zhen X, O’Tuathaigh CMP. Developmental Genes and Regulatory Proteins, Domains of Cognitive Impairment in Schizophrenia Spectrum Psychosis and Implications for Antipsychotic Drug Discovery: The Example of Dysbindin-1 Isoforms and Beyond. Front Pharm. 2019;10:1638.
Straub RE, MacLean CJ, O’Neill FA, Burke J, Murphy B, Duke F, et al. A potential vulnerability locus for schizophrenia on chromosome 6p24-22: evidence for genetic heterogeneity. Nat Genet. 1995;11:287–93.
Talbot K, Ong WY, Blake DJ, Tang J, Louneva N, Carlson GC et al. Dysbindin-1 and Its Protein Family. In: Javitt DC, Kantrowitz J, editors. Handbook of Neurochemistry and Molecular Neurobiology, 3rd edn. New York:Springer Science;2009. p. 107–241.
Marshall CR, Howrigan DP, Merico D, Thiruvahindrapuram B, Wu W, Greer DS, et al. Contribution of copy number variants to schizophrenia from a genome-wide study of 41,321 subjects. Nat Genet. 2017;49:27–35.
Pae CU, Drago A, Kim JJ, Patkar AA, Jun TY, Lee C, et al. DTNBP1 haplotype influences baseline assessment scores of schizophrenic in-patients. Neurosci Lett. 2008;440:150–4.
Bray NJ, Preece A, Williams NM, Moskvina V, Buckland PR, Owen MJ, et al. Haplotypes at the dystrobrevin binding protein 1 (DTNBP1) gene locus mediate risk for schizophrenia through reduced DTNBP1 expression. Hum Mol Genet. 2005;14:1947–54.
Straub RE, Jiang Y, MacLean CJ, Ma Y, Webb BT, Myakishev MV, et al. Genetic variation in the 6p22.3 gene DTNBP1, the human ortholog of the mouse dysbindin gene, is associated with schizophrenia. Am J Hum Genet. 2002;71:337–48.
Voisey J, Swagell CD, Hughes IP, Lawford BR, Young RM, Morris CP. Analysis of HapMap tag-SNPs in dysbindin (DTNBP1) reveals evidence of consistent association with schizophrenia. Eur Psychiatry. 2010;25:314–9.
Talbot K, Eidem WL, Tinsley CL, Benson MA, Thompson EW, Smith RJ, et al. Dysbindin-1 is reduced in intrinsic, glutamatergic terminals of the hippocampal formation in schizophrenia. J Clin Investig. 2004;113:1353–63.
Weickert CS, Straub RE, McClintock BW, Matsumoto M, Hashimoto R, Hyde TM, et al. Human dysbindin (DTNBP1) gene expression in normal brain and in schizophrenic prefrontal cortex and midbrain. Arch Gen Psychiatry. 2004;61:544–55.
Papaleo F, Weinberger DR. Dysbindin and Schizophrenia: it’s dopamine and glutamate all over again. Biol Psychiatry. 2011;69:2–4.
Tang J, LeGros RP, Louneva N, Yeh L, Cohen JW, Hahn CG, et al. Dysbindin-1 in dorsolateral prefrontal cortex of schizophrenia cases is reduced in an isoform-specific manner unrelated to dysbindin-1 mRNA expression. Hum Mol Genet. 2009;18:3851–63.
Wentzel C, Delvendahl I, Sydlik S, Georgiev O, Muller M. Dysbindin links presynaptic proteasome function to homeostatic recruitment of low release probability vesicles. Nat Commun. 2018;9:267.
Ji Y, Yang F, Papaleo F, Wang HX, Gao WJ, Weinberger DR, et al. Role of dysbindin in dopamine receptor trafficking and cortical GABA function. Proc Natl Acad Sci USA. 2009;106:19593–8.
Shao L, Shuai Y, Wang J, Feng S, Lu B, Li Z, et al. Schizophrenia susceptibility gene dysbindin regulates glutamatergic and dopaminergic functions via distinctive mechanisms in Drosophila. Proc Natl Acad Sci USA. 2011;108:18831–6.
Shao W, Zhang SZ, Tang M, Zhang XH, Zhou Z, Yin YQ, et al. Suppression of neuroinflammation by astrocytic dopamine D2 receptors via alphaB-crystallin. Nature. 2013;494:90–4.
Cui Q, Pitt JE, Pamukcu A, Poulin JF, Mabrouk OS, Fiske MP, et al. Blunted mGluR Activation Disinhibits Striatopallidal Transmission in Parkinsonian Mice. Cell Rep. 2016;17:2431–44.
Petrelli F, Dallérac G, Pucci L, Calì C, Zehnder T, Sultan S, et al. Dysfunction of homeostatic control of dopamine by astrocytes in the developing prefrontal cortex leads to cognitive impairments. Mol Psychiatry. 2020;25:732–49. https://doi.org/10.1038/s41380-018-0226-y.
Corkrum M, Covelo A, Lines J, Bellocchio L, Pisansky M, Loke K, et al. Dopamine-Evoked Synaptic Regulation in the Nucleus Accumbens Requires Astrocyte Activity. Neuron. 2020;105:1036–47.e5. https://doi.org/10.1016/j.neuron.2019.12.026. Epub 2020 Jan 15.
Talbot K, Louneva N, Cohen JW, Kazi H, Blake DJ, Arnold SE. Synaptic dysbindin-1 reductions in schizophrenia occur in an isoform-specific manner indicating their subsynaptic location. PLoS ONE. 2011;6:e16886.
Larimore J, Zlatic SA, Gokhale A, Tornieri K, Singleton KS, Mullin AP, et al. Mutations in the BLOC-1 subunits dysbindin and muted generate divergent and dosage-dependent phenotypes. J Biol Chem. 2014;289:14291–300.
Yuan Y, Wang H, Wei Z, Li W. Impaired autophagy in hilar mossy cells of the dentate gyrus and its implication in schizophrenia. J Genet Genom. 2015;42:1–8.
Petit EI, Michalak Z, Cox R, O’Tuathaigh CM, Clarke N, Tighe O, et al. Dysregulation of Specialized Delay/Interference-Dependent Working Memory Following Loss of Dysbindin-1A in Schizophrenia-Related Phenotypes. Neuropsychopharmacology. 2017;42:1349–60.
Mori T, Tanaka K, Buffo A, Wurst W, Kuhn R, Gotz M. Inducible gene deletion in astroglia and radial glia-a valuable tool for functional and lineage analysis. Glia. 2006;54:21–34.
Scheggia D, Zamberletti E, Realini N, Mereu M, Contarini G, Ferretti V, et al. Remote memories are enhanced by COMT activity through dysregulation of the endocannabinoid system in the prefrontal cortex. Mol Psychiatry. 2018;23:1040–50.
Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. Amsterdam:Elsevier Academic Press;2004.
Dugue GP, Dumoulin A, Triller A, Dieudonne S. Target-dependent use of co-released inhibitory transmitters at central synapses. J Neurosci. 2005;25:6490–8.
Srinivasan R, Huang BS, Venugopal S, Johnston AD, Chai H, Zeng H, et al. Ca(2+) signaling in astrocytes from Ip3r2(-/-) mice in brain slices and during startle responses in vivo. Nat Neurosci. 2015;18:708–17.
Mariotti L, Losi G, Lia A, Melone M, Chiavegato A, Gomez-Gonzalo M, et al. Interneuron-specific signaling evokes distinctive somatostatin-mediated responses in adult cortical astrocytes. Nat Commun. 2018;9:82.
Caffino L, Verheij MMM, Roversi K, Targa G, Mottarlini F, Popik P, et al. Hypersensitivity to amphetamine’s psychomotor and reinforcing effects in serotonin transporter knockout rats: Glutamate in the nucleus accumbens. Br J Pharm. 2020;177:4532–47.
Huang H, Michetti C, Busnelli M, Manago F, Sannino S, Scheggia D, et al. Chronic and acute intranasal oxytocin produce divergent social effects in mice. Neuropsychopharmacology. 2014;39:1102–14.
Scheggia D, Bebensee A, Weinberger DR, Papaleo F. The ultimate intra-/extra-dimensional attentional set-shifting task for mice. Biol Psychiatry. 2014;75:660–70.
Manago F, Mereu M, Mastwal S, Mastrogiacomo R, Scheggia D, Emanuele M, et al. Genetic Disruption of Arc/Arg3.1 in Mice Causes Alterations in Dopamine and Neurobehavioral Phenotypes Related to Schizophrenia. Cell Rep. 2016;16:2116–28.
Papaleo F, Kieffer BL, Tabarin A, Contarino A. Decreased motivation to eat in mu-opioid receptor-deficient mice. Eur J Neurosci. 2007;25:3398–405.
Polishchuk RS, Mironov AA. Correlative video light/electron microscopy. Curr Protoc Cell Biol. 2001;Chapter 4:Unit 4.8. https://doi.org/10.1002/0471143030.cb0408s11.
Luskin MB, Parnavelas JG, Barfield JA. Neurons, astrocytes, and oligodendrocytes of the rat cerebral cortex originate from separate progenitor cells: an ultrastructural analysis of clonally related cells. J Neurosci. 1993;13:1730–50.
Nahirney PC, Tremblay ME. Brain ultrastructure: putting the pieces together. Front Cell Dev Biol. 2021;9:629503.
Orso G, Pendin D, Liu S, Tosetto J, Moss TJ, Faust JE, et al. Homotypic fusion of ER membranes requires the dynamin-like GTPase atlastin. Nature. 2009;460:978–83.
De Filippis C, Napoli B, Rigon L, Guarato G, Bauer R, Tomanin R et al. Drosophila D-idua Reduction Mimics Mucopolysaccharidosis Type I Disease-Related Phenotypes. Cells 2021;11:129. https://doi.org/10.3390/cells11010129.
Scheggia D, Manago F, Maltese F, Bruni S, Nigro M, Dautan D, et al. Somatostatin interneurons in the prefrontal cortex control affective state discrimination in mice. Nat Neurosci. 2020;23:47–60.
Zamanian JL, Xu L, Foo LC, Nouri N, Zhou L, Giffard RG, et al. Genomic analysis of reactive astrogliosis. J Neurosci. 2012;32:6391–410.
Ferretti V, Maltese F, Contarini G, Nigro M, Bonavia A, Huang H, et al. Oxytocin Signaling in the Central Amygdala Modulates Emotion Discrimination in Mice. Curr Biol. 2019;29:1938–53 e1936.
Papaleo F, Yang F, Garcia S, Chen J, Lu B, Crawley JN, et al. Dysbindin-1 modulates prefrontal cortical activity and schizophrenia-like behaviors via dopamine/D2 pathways. Mol Psychiatry. 2012;17:85–98.
Feng YQ, Zhou ZY, He X, Wang H, Guo XL, Hao CJ, et al. Dysbindin deficiency in sandy mice causes reduction of snapin and displays behaviors related to schizophrenia. Schizophrenia Res. 2008;106:218–28.
Hattori S, Murotani T, Matsuzaki S, Ishizuka T, Kumamoto N, Takeda M, et al. Behavioral abnormalities and dopamine reductions in sdy mutant mice with a deletion in Dtnbp1, a susceptibility gene for schizophrenia. Biochem Biophys Res Commun. 2008;373:298–302.
Robbins TW. Shifting and stopping: fronto-striatal substrates, neurochemical modulation and clinical implications. Philos Trans R Soc Lond. 2007;362:917–32.
Clarke HF, Hill GJ, Robbins TW, Roberts AC. Dopamine, but not serotonin, regulates reversal learning in the marmoset caudate nucleus. J Neurosci. 2011;31:4290–7.
Doherty JM, Masten VL, Powell SB, Ralph RJ, Klamer D, Low MJ, et al. Contributions of dopamine D1, D2, and D3 receptor subtypes to the disruptive effects of cocaine on prepulse inhibition in mice. Neuropsychopharmacology. 2008;33:2648–56.
Koch M. The neurobiology of startle. Prog Neurobiol. 1999;59:107–28.
Plappert CF, Pilz PK, Schnitzler HU. Factors governing prepulse inhibition and prepulse facilitation of the acoustic startle response in mice. Behav Brain Res. 2004;152:403–12.
Drew MR, Simpson EH, Kellendonk C, Herzberg WG, Lipatova O, Fairhurst S, et al. Transient overexpression of striatal D2 receptors impairs operant motivation and interval timing. J Neurosci. 2007;27:7731–9.
Ward RD, Kellendonk C, Kandel ER, Balsam PD. Timing as a window on cognition in schizophrenia. Neuropharmacology. 2012;62:1175–81. https://doi.org/10.1016/j.neuropharm.2011.04.014. Epub 2011 Apr 21.
Ward RD, Kellendonk C, Simpson EH, Lipatova O, Drew MR, Fairhurst S, et al. Impaired timing precision produced by striatal D2 receptor overexpression is mediated by cognitive and motivational deficits. Behav Neurosci. 2009;123:720–30.
Marley A, von Zastrow M. Dysbindin promotes the post-endocytic sorting of G protein-coupled receptors to lysosomes. PLoS ONE. 2010;5:e9325.
Leggio GM, Torrisi SA, Mastrogiacomo R, Mauro D, Chisari M, Devroye C, et al. The epistatic interaction between the dopamine D3 receptor and dysbindin-1 modulates higher-order cognitive functions in mice and humans. Mol Psychiatry. 2021;26:1272–85.
Bucci C, Parton RG, Mather IH, Stunnenberg H, Simons K, Hoflack B, et al. The small GTPase rab5 functions as a regulatory factor in the early endocytic pathway. Cell. 1992;70:715–28.
Soldati T, Rancano C, Geissler H, Pfeffer SR. Rab7 and Rab9 are recruited onto late endosomes by biochemically distinguishable processes. J Biol Chem. 1995;270:25541–8.
Liu X, Cheng C, Shao B, Wu X, Ji Y, Lu X, et al. The functional interaction between CDK11p58 and beta-1,4-galactosyltransferase I involved in astrocyte activation caused by lipopolysaccharide. Inflammation. 2012;35:1365–77.
Nakamura N, Rabouille C, Watson R, Nilsson T, Hui N, Slusarewicz P, et al. Characterization of a cis-Golgi matrix protein, GM130. J Cell Biol. 1995;131:1715–26.
Santello M, Toni N, Volterra A. Astrocyte function from information processing to cognition and cognitive impairment. Nat Neurosci. 2019;22:154–66.
Papaleo F, Burdick MC, Callicott JH, Weinberger DR. Epistatic interaction between COMT and DTNBP1 modulates prefrontal function in mice and in humans. Mol Psychiatry. 2014;19:311–6.
Weinstein JJ, Chohan MO, Slifstein M, Kegeles LS, Moore H, Abi-Dargham A. Pathway-Specific Dopamine Abnormalities in Schizophrenia. Biol Psychiatry. 2017;81:31–42.
Winterer G, Weinberger DR. Genes, dopamine and cortical signal-to-noise ratio in schizophrenia. Trends Neurosci. 2004;27:683–90.
Simpson EH, Kellendonk C. Insights About Striatal Circuit Function and Schizophrenia From a Mouse Model of Dopamine D2 Receptor Upregulation. Biol Psychiatry. 2017;81:21–30.
Chai H, Diaz-Castro B, Shigetomi E, Monte E, Octeau JC, Yu X, et al. Neural Circuit-Specialized Astrocytes: Transcriptomic, Proteomic, Morphological, and Functional Evidence. Neuron. 2017;95:531–49 e539.
Ben Haim L, Rowitch DH. Functional diversity of astrocytes in neural circuit regulation. Nat Rev Neurosci. 2017;18:31–41.
Huang AY, Woo J, Sardar D, Lozzi B, Bosquez Huerta NA, Lin CJ, et al. Region-Specific Transcriptional Control of Astrocyte Function Oversees Local Circuit Activities. Neuron. 2020;106:992–1008 e1009.
Mohebi A, Pettibone JR, Hamid AA, Wong JT, Vinson LT, Patriarchi T, et al. Dissociable dopamine dynamics for learning and motivation. Nature. 2019;570:65–70.
Takeuchi Y, Yamamoto H, Fukunaga K, Miyakawa T, Miyamoto E. Identification of the isoforms of Ca(2+)/Calmodulin-dependent protein kinase II in rat astrocytes and their subcellular localization. J Neurochem. 2000;74:2557–67.
Roberts BM, Doig NM, Brimblecombe KR, Lopes EF, Siddorn RE, Threlfell S, et al. GABA uptake transporters support dopamine release in dorsal striatum with maladaptive downregulation in a parkinsonism model. Nat Commun. 2020;11:4958.
Gittis AH, Berke JD, Bevan MD, Chan CS, Mallet N, Morrow MM, et al. New roles for the external globus pallidus in basal ganglia circuits and behavior. J Neurosci. 2014;34:15178–83.
Carvalho Poyraz F, Holzner E, Bailey MR, Meszaros J, Kenney L, Kheirbek MA, et al. Decreasing Striatopallidal Pathway Function Enhances Motivation by Energizing the Initiation of Goal-Directed Action. J Neurosci. 2016;36:5988–6001.
Fiore VG, Nolte T, Rigoli F, Smittenaar P, Gu X, Dolan RJ. Value encoding in the globus pallidus: fMRI reveals an interaction effect between reward and dopamine drive. Neuroimage. 2018;173:249–57.
Miller JM, Vorel SR, Tranguch AJ, Kenny ET, Mazzoni P, van Gorp WG, et al. Anhedonia after a selective bilateral lesion of the globus pallidus. Am J Psychiatry. 2006;163:786–8.
Sotoyama H, Zheng Y, Iwakura Y, Mizuno M, Aizawa M, Shcherbakova K, et al. Pallidal hyperdopaminergic innervation underlying D2 receptor-dependent behavioral deficits in the schizophrenia animal model established by EGF. PLoS ONE. 2011;6:e25831.
Avila G, Picazo O, Chuc-Meza E, Garcia-Ramirez M. Reduction of dopaminergic transmission in the globus pallidus increases anxiety-like behavior without altering motor activity. Behav Brain Res. 2020;386:112589.
Bartlett SE, Enquist J, Hopf FW, Lee JH, Gladher F, Kharazia V, et al. Dopamine responsiveness is regulated by targeted sorting of D2 receptors. Proc Natl Acad Sci USA. 2005;102:11521–6.
Thompson D, Whistler JL. Dopamine D(3) receptors are down-regulated following heterologous endocytosis by a specific interaction with G protein-coupled receptor-associated sorting protein-1. J Biol Chem. 2011;286:1598–608.
Chazalon M, Paredes-Rodriguez E, Morin S, Martinez A, Cristovao-Ferreira S, Vaz S, et al. GAT-3 Dysfunction Generates Tonic Inhibition in External Globus Pallidus Neurons in Parkinsonian Rodents. Cell Rep. 2018;23:1678–90.
Adermark L, Lagström O, Loftén A, Licheri V, Havenäng A, Loi EA, et al. Astrocytes modulate extracellular neurotransmitter levels and excitatory neurotransmission in dorsolateral striatum via dopamine D2 receptor signaling. Neuropsychopharmacology. 2022;47:1493–1502. https://doi.org/10.1038/s41386-021-01232-x. Epub 2021 Nov 22.
Jiang M, Chen G. Ca2+ regulation of dynamin-independent endocytosis in cortical astrocytes. J Neurosci. 2009;29:8063–74.
Boulay AC, Saubamea B, Adam N, Chasseigneaux S, Mazare N, Gilbert A, et al. Translation in astrocyte distal processes sets molecular heterogeneity at the gliovascular interface. Cell Disco. 2017;3:17005.
Xin H, Wang F, Li Y, Lu QE, Cheung WL, Zhang Y, et al. Secondary Release of Exosomes From Astrocytes Contributes to the Increase in Neural Plasticity and Improvement of Functional Recovery After Stroke in Rats Treated With Exosomes Harvested From MicroRNA 133b-Overexpressing Multipotent Mesenchymal Stromal Cells. Cell Transpl. 2017;26:243–57.
Acknowledgements
We thank M. Morini, E. Albanesi, D. Cantatore, G. Pruzzo, T. Catelani, D. Mauro, S. Monari, F. Torri, B. Chiarenza, A. Monteforte and C. Chiabrera, for technical support. We thank GlaxoSmithKline and Dr S. Wilson for generously donating the newly generated Dys1Aflox mice. We thank Dr D.R. Weinberger and Dr R.E. Straub for initial access to the Brain Cloud databank. This work was supported by funding from the Marie Sklodowska-Curie Fellowships (grant n.796244) to CD; the Ministero dell’Universita’ e della Ricerca italiano (project PRIN 2017K2NEF4) to FD; grant MIUR Progetto eccellenza to FF; the Istituto Italiano di Tecnologia, the Brain and Behavior Research Foundation (2015 NARSAD grant n.23234), the Ministero della Salute italiano (project GR-2016-02362413), and Fondazione Telethon Italia (project GGP19103) to FP.
Author information
Authors and Affiliations
Contributions
Conceptualization, RM, GT, and FP; Methodology and Investigation, RM, GT, DD, CD, GL, AL, LC, FF, VF, GO, RM, FM, GP, AF, GC, MADL, FM, and FP; Resources, GO, DR, JLW, GML, GC, CSW, and FP; Writing, all authors; Visualization, RM, GT, DD, CD, GL, AL, GL, GO, RM, FM, and FP; Supervision, FP; Funding Acquisition, FD, CSW, and FP.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Mastrogiacomo, R., Trigilio, G., Devroye, C. et al. Dysbindin-1A modulation of astrocytic dopamine and basal ganglia dependent behaviors relevant to schizophrenia. Mol Psychiatry 27, 4201–4217 (2022). https://doi.org/10.1038/s41380-022-01683-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-022-01683-8