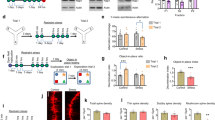
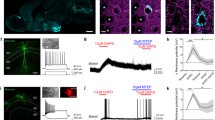
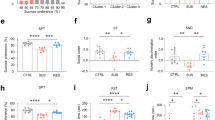
Abstract
Reduced somatostatin (SST) and dysfunction of SST-positive (SST+) neurons are hallmarks of neurological disorders and associated with mood disturbances, but the molecular origin of SST+ neuron vulnerability is unknown. Using chronic psychosocial stress as a paradigm to induce elevated behavioral emotionality in rodents, we report a selective vulnerability of SST+ neurons through exacerbated unfolded protein response (UPR) of the endoplasmic reticulum (ER), or ER stress, in the prefrontal cortex. We next show that genetically suppressing ER stress in SST+ neurons, but not in pyramidal neurons, normalized behavioral emotionality induced by psychosocial stress. In search for intrinsic factors mediating SST+ neuron vulnerability, we found that the forced expression of the SST precursor protein (preproSST) in SST+ neurons, mimicking psychosocial stress-induced early proteomic changes, induces ER stress, whereas mature SST or processing-incompetent preproSST does not. Biochemical analyses further show that psychosocial stress induces SST protein aggregation under elevated ER stress conditions. These results demonstrate that SST processing in the ER is a SST+ neuron-intrinsic vulnerability factor under conditions of sustained or over-activated UPR, hence negatively impacting SST+ neuron functions. Combined with observations in major medical illness, such as diabetes, where excess ER processing of preproinsulin similarly causes ER stress and β cell dysfunction, this suggests a universal mechanism for proteinopathy that is induced by excess processing of native endogenous proteins, playing critical pathophysiological roles that extend to neuropsychiatric disorders.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Epelbaum J, Guillou J-L, Gastambide F, Hoyer D, Duron E, Viollet C. Somatostatin, Alzheimer’s disease and cognition: an old story coming of age? Prog Neurobiol. 2009;89:153–61.
Hendry SH, Jones EG, Emson PC. Morphology, distribution, and synaptic relations of somatostatin- and neuropeptide Y-immunoreactive neurons in rat and monkey neocortex. J Neurosci. 1984;4:2497–517.
Melchitzky DS, Lewis DA. Dendritic-targeting GABA neurons in monkey prefrontal cortex: comparison of somatostatin- and calretinin-immunoreactive axon terminals. Synapse. 2008;62:456–65.
Xu X, Roby KD, Callaway EM. Immunochemical characterization of inhibitory mouse cortical neurons: three chemically distinct classes of inhibitory cells. J Comp Neurol. 2010;518:389–404.
Soumier A, Sibille E. Opposing effects of acute versus chronic blockade of frontal cortex somatostatin-positive inhibitory neurons on behavioral emotionality in mice. Neuropsychopharmacology. 2014;39:2252–62.
Lin L, Sibille E. Somatostatin, neuronal vulnerability and behavioral emotionality. Mol Psychiatry. 2015;20:377–87.
Fee C, Prevot TD, Misquitta K, Knutson DE, Li G, Mondal P, et al. Behavioral deficits induced by somatostatin-positive GABA neuron silencing are rescued by alpha 5 GABA-A receptor potentiation. Int J Neuropsychopharmacol. 2021; pyab002.
Martel G, Dutar P, Epelbaum J, Viollet C. Somatostatinergic systems: an update on brain functions in normal and pathological aging. Front Endocrinol. 2012;3:154.
Fee C, Banasr M, Sibille E. Somatostatin-positive gamma-aminobutyric acid interneuron deficits in depression: cortical microcircuit and therapeutic perspectives. Biol Psychiatry. 2017;82:549–59.
Prévot T, Sibille E. Altered GABA-mediated information processing and cognitive dysfunctions in depression and other brain disorders. Mol Psychiatry. 2021;26:151–67.
Willner P. The chronic mild stress (CMS) model of depression: History, evaluation and usage. Neurobiol Stress. 2016;6:78–93.
Tripp A, Kota RS, Lewis DA, Sibille E. Reduced somatostatin in subgenual anterior cingulate cortex in major depression. Neurobiol Dis. 2011;42:116–24.
Guilloux JP, Douillard-Guilloux G, Kota R, Wang X, Gardier AM, Martinowich K, et al. Molecular evidence for BDNF- and GABA-related dysfunctions in the amygdala of female subjects with major depression. Mol Psychiatry. 2012;17:1130–42.
Girgenti MJ, Wohleb ES, Mehta S, Ghosal S, Fogaca MV, Duman RS. Prefrontal cortex interneurons display dynamic sex-specific stress-induced transcriptomes. Transl Psychiatry. 2019;9:292.
Ron D, Harding HP. Protein-folding homeostasis in the endoplasmic reticulum and nutritional regulation. Cold Spring Harb Perspect Biol. 2012;4:a013177.
Metcalf MG, Higuchi-Sanabria R, Garcia G, Tsui CK, Dillin A. Beyond the cell factory: Homeostatic regulation of and by the UPRER. Sci Adv. 2020;6:eabb9614.
Gonen N, Sabath N, Burge CB, Shalgi R. Widespread PERK-dependent repression of ER targets in response to ER stress. Sci Rep. 2019;9:4330.
Gerakis Y, Hetz C. Emerging roles of ER stress in the etiology and pathogenesis of Alzheimer’s disease. FEBS J. 2018;285:995–1011.
Rozpedek W, Markiewicz L, Diehl JA, Pytel D, Majsterek I. Unfolded protein response and PERK kinase as a new therapeutic target in the pathogenesis of Alzheimer’s disease. Curr Med Chem. 2015;22:3169–84.
Hetz C, Saxena S. ER stress and the unfolded protein response in neurodegeneration. Nat Rev Neurol. 2017;13:477–91.
Arunagiri A, Haataja L, Cunningham CN, Shrestha N, Tsai B, Qi L, Liu M, Arvan P. Misfolded proinsulin in the endoplasmic reticulum during development of beta cell failure in diabetes. Ann NY Acad Sci. 2018;1418:5–19.
Negro-Vilar A, Saavedra JM. Changes in brain somatostatin and vasopressin levels after stress in spontaneously hypertensive and Wistar-Kyoto rats. Brain Res Bull. 1980;5:353–8.
Arancibia S, Epelbaum J, Boyer R, Assenmacher I. In vivo release of somatostatin from rat median eminence after local K+ infusion or delivery of nociceptive stress. Neurosci Lett. 1984;50:97–102.
Arancibia S, Rage F, Grauges P, Gomez F, Tapia-Arancibia L, Armario A. Rapid modifications of somatostatin neuron activity in the periventricular nucleus after acute stress. Exp Brain Res. 2000;134:261–7.
Chen XQ, Du JZ. Increased somatostatin mRNA expression in periventricular nucleus of rat hypothalamus during hypoxia. Regul Pept. 2002;105:197–201.
Priego T, Ibanez De Caceres I, Martin AI, Villanua MA, Lopez-Calderon A. Endotoxin administration increases hypothalamic somatostatin mRNA through nitric oxide release. Regul Pept. 2005;124:113–8.
Polkowska J, Wankowska M. Effects of maternal deprivation on the somatotrophic axis and neuropeptide Y in the hypothalamus and pituitary in female lambs. The histomorphometric study. Folia Histochem Cytobiol. 2010;48:299–305.
Prévôt TD, Gastambide F, Viollet C, Henkous N, Martel G, Epelbaum J, Béracochéa D, Guillou J-L. Roles of hippocampal somatostatin receptor subtypes in stress response and emotionality. Neuropsychopharmacology. 2017;42:1647–56.
Goodman RH, Aron DC, Roos BA. Rat pre-prosomatostatin. Struct Process microsomal Membr J Biol Chem. 1983;258:5570–3.
Newton DF, Oh H, Shukla R, Misquitta K, Fee C, Banasr M, et al. Chronic stress induces coordinated cortical microcircuit cell-type transcriptomic changes consistent with altered information processing. Biol Psychiatry. 2021; https://doi.org/10.1101/2020.08.18.249995.
Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102:15545–50.
van Waarde-Verhagen M, Kampinga HH. Measurement of chaperone-mediated effects on polyglutamine protein aggregation by the filter trap assay. Methods Mol Biol. 2018;1709:59–74.
Nucifora LG, MacDonald ML, Lee BJ, Peters ME, Norris AL, Orsburn BC, et al. Increased protein insolubility in brains from a subset of patients with schizophrenia. Am J Psychiatry. 2019;176:730–43.
Hui KK, Takashima N, Watanabe A, Chater TE, Matsukawa H, Nekooki-Machida Y, Nilsson P, Endo R, Goda Y, Saido TC, Yoshikawa T, Tanaka M. GABARAPs dysfunction by autophagy deficiency in adolescent brain impairs GABAA receptor trafficking and social behavior. Sci Adv. 2019;5:eaau8237.
Guilloux JP, Seney M, Edgar N, Sibille E. Integrated behavioral Z-scoring increases the sensitivity and reliability of behavioral phenotyping in mice: relevance to emotionality and sex. J Neurosci Methods. 2011;197:21–31.
Seney ML, Huo Z, Cahill K, French L, Puralewski R, Zhang J, Logan RW, Tseng G, Lewis DA, Sibille E. Opposite molecular signatures of depression in men and women. Biol Psychiatry. 2018;84:18–27.
Riekkinen PJ, Pitkänen A. Somatostatin and epilepsy. Metabolism. 1990;39:112–5.
Solarski M, Wang H, Wille H, Schmitt-Ulms G. Somatostatin in Alzheimer’s disease: a new role for an old player. Prion. 2018;12:1–8.
Maji SK, Perrin MH, Sawaya MR, Jessberger S, Vadodaria K, Rissman RA, et al. Functional amyloids as natural storage of peptide hormones in pituitary secretory granules. Science. 2009;325:328–32.
Bradshaw NJ, Korth C. Protein misassembly and aggregation as potential convergence points for non-genetic causes of chronic mental illness. Mol Psychiatry. 2019;24:936–51.
Sumitomo A, Yukitake H, Hirai K, Horike K, Ueta K, Chung Y, et al. Ulk2 controls cortical excitatory-inhibitory balance via autophagic regulation of p62 and GABAA receptor trafficking in pyramidal neurons. Hum Mol Genet. 2018;27:3165–76.
Bown C, Wang JF, MacQueen G, Young LT. Increased temporal cortex ER stress proteins in depressed subjects who died by suicide. Neuropsychopharmacology. 2000;22:327–32.
Wang H, Muiznieks LD, Ghosh P, Williams D, Solarski M, Fang A, Ruiz-Riquelme A, Pomès R, Watts JC, Chakrabartty A, Wille H, Sharpe S, Schmitt-Ulms G. Somatostatin binds to the human amyloid β peptide and favors the formation of distinct oligomers. Elife. 2017;6:e28401. pii
Sharma V, Sood R, Khlaifia A, Eslamizade MJ, Hung TY, Lou D, et al. eIF2α controls memory consolidation via excitatory and somatostatin neurons. Nature. 2020;586:412–6.
Liu M, Sun J, Cui J, Chen W, Guo H, Barbetti F, Arvan P. INS-gene mutations: from genetics and beta cell biology to clinical disease. Mol Asp Med. 2015;42:3–18.
Acknowledgements
The authors thank Mohan Pabba, Rammohan Shukla, Mounira Banasr, Thomas Prevot, and Hyunjung Oh for comments or discussion. This work was supported by grants from the Canadian Institute of Health Research (CIHR #153175 to ES), National Alliance for Research on Schizophrenia and Depression (NARSAD award #25637 to ES), the National Institutes of Health (MH-093723 to ES), Campbell Family Mental Health Research Institute (to ES).
Author information
Authors and Affiliations
Contributions
TT and ES conceived the study and designed the experiments; TT and AS acquired and analyzed data; DN analyzed gene expression profiles; TT and ES wrote and edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Tomoda, T., Sumitomo, A., Newton, D. et al. Molecular origin of somatostatin-positive neuron vulnerability. Mol Psychiatry 27, 2304–2314 (2022). https://doi.org/10.1038/s41380-022-01463-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-022-01463-4