Abstract
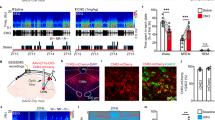
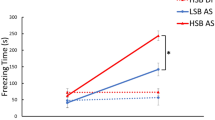
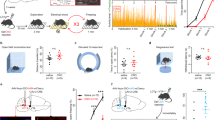
The amygdala, a critical brain region responsible for emotional behavior, is crucially involved in the regulation of the effects of stress on emotional behavior. In the mammalian forebrain, gastrin-releasing peptide (GRP), a 27-amino-acid mammalian neuropeptide, which is a homolog of the 14-amino-acid amidated amphibian peptide bombesin, is highly expressed in the amygdala. The levels of GRP are markedly increased in the amygdala after acute stress; therefore, it is known as a stress-activated modulator. To determine the role of GRP in emotional behavior under stress, we conducted some behavioral and biochemical experiments with GRP-knockout (KO) mice. GRP-KO mice exhibited a longer freezing response than wild-type (WT) littermates in both contextual and auditory fear (also known as threat) conditioning tests only when they were subjected to acute restraint stress 20 min before the conditioning. To identify the critical neural circuits associated with the regulation of emotional memory by GRP, we conducted Arc/Arg3.1-reporter mapping in the amygdala with an Arc-Venus reporter transgenic mouse line. In the amygdalostriatal transition area (AST) and the lateral side of the basal nuclei, fear conditioning after restraint stress increased neuronal activity significantly in WT mice, and GRP KO was found to negate this potentiation only in the AST. These results indicate that the GRP-activated neurons in the AST are likely to suppress excessive fear expression through the regulation of downstream circuits related to fear learning following acute stress.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Goligorsky MS. The concept of cellular “fight-or-flight” reaction to stress. Am J Physiol Ren Physiol. 2001;280:F551–61.
Deppermann S, Storchak H, Fallgatter AJ, Ehlis AC. Stress-induced neuroplasticity: (mal)adaptation to adverse life events in patients with PTSD−a critical overview. Neuroscience. 2014;283:166–77.
Grillon C, Duncko R, Covington MF, Kopperman L, Kling MA. Acute stress potentiates anxiety in humans. Biol Psychiatry. 2007;62:1183–86.
Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology 2010;35:169–91.
Cisler JM, Olatunji BO, Feldner MT, Forsyth JP. Emotion regulation and the anxiety disorders: an integrative review. J Psychopathol Behav Assess. 2010;32:68–82.
Duvarci S, Pare D. Amygdala Microcircuits Controlling Learned Fear. Neuron 2014;82:966–80.
LeDoux JE. Emotion circuits in the brain. Annu Rev Nneurosci. 2000;23:155–84.
Nanda SA, Qi C, Roseboom PH, Kalin NH. Predator stress induces behavioral inhibition and amygdala somatostatin receptor 2 gene expression. Genes Brain Behav. 2008;7:639–48.
Kolber BJ, Roberts MS, Howell MP, Wozniak DF, Sands MS, Muglia LJ. Central amygdala glucocorticoid receptor action promotes fear-associated CRH activation and conditioning. Proc Natl Acad Sci USA. 2008;105:12004–9.
Wang XY, Zhao M, Ghitza UE, Li YQ, Lu L. Stress impairs reconsolidation of drug memory via glucocorticoid receptors in the basolateral amygdala. J Neurosci. 2008;28:5602–10.
Morilak DA, Barrera G, Echevarria DJ, Garcia AS, Hernandez A, Ma S, et al. Role of brain norepinephrine in the behavioral response to stress. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1214–24.
Johnson LR, Hou M, Prager EM, Ledoux JE. Regulation of the Fear Network by Mediators of Stress: Norepinephrine Alters the Balance between Cortical and Subcortical Afferent Excitation of the Lateral Amygdala. Front Behav Neurosci. 2011;5:23.
McCall JG, Al-Hasani R, Siuda ER, Hong DY, Norris AJ, Ford CP, et al. CRH Engagement of the Locus Coeruleus Noradrenergic System Mediates Stress-Induced Anxiety. Neuron 2015;87:605–20.
Inoue R, Abdou K, Hayashi-Tanaka A, Muramatsu SI, Mino K, Inokuchi K, et al. Glucocorticoid receptor-mediated amygdalar metaplasticity underlies adaptive modulation of fear memory by stress. eLife 2018;7:e34135.
Gutierrez-Mariscal M, Sanchez E, Rebolledo-Solleiro D, Garcia-Vazquez AI, Cote-Velez A, Acasuso-Rivero C, et al. The acute response of the amygdalar TRH system to psychogenic stressors varies dependent on the paradigm and circadian condition. Brain Res. 2012;1452:73–84.
Ischia J, Patel O, Shulkes A, Baldwin GS. Gastrin-releasing peptide: different forms, different functions. BioFactors 2009;35:69–75.
McDonald TJ, Jornvall H, Nilsson G, Vagne M, Ghatei M, Bloom SR, et al. Characterization of a gastrin releasing peptide from porcine non-antral gastric tissue. Biochem Biophys Res Commun. 1979;90:227–33.
Jensen RT, Battey JF, Spindel ER, Benya RV. International Union of Pharmacology. LXVIII. Mammalian bombesin receptors: nomenclature, distribution, pharmacology, signaling, and functions in normal and disease states. Pharm Rev. 2008;60:1–42.
Chaperon F, Fendt M, Kelly PH, Lingenhoehl K, Mosbacher J, Olpe HR, et al. Gastrin-releasing peptide signaling plays a limited and subtle role in amygdala physiology and aversive memory. PLoS ONE. 2012;7:e34963.
Kamichi S, Wada E, Aoki S, Sekiguchi M, Kimura I, Wada K. Immunohistochemical localization of gastrin-releasing peptide receptor in the mouse brain. Brain Res. 2005;1032:162–70.
Hermes ML, Kolaj M, Coderre EM, Renaud LP. Gastrin-releasing peptide acts via postsynaptic BB2 receptors to modulate inward rectifier K+ and TRPV1-like conductances in rat paraventricular thalamic neurons. J Physiol. 2013;591:1823–39.
Gamble KL, Kudo T, Colwell CS, McMahon DG. Gastrin-releasing peptide modulates fast delayed rectifier potassium current in Per1-expressing SCN neurons. J Biol Rhythms. 2011;26:99–106.
van den Pol AN, Yao Y, Fu LY, Foo K, Huang H, Coppari R, et al. Neuromedin B and gastrin-releasing peptide excite arcuate nucleus neuropeptide Y neurons in a novel transgenic mouse expressing strong Renilla green fluorescent protein in NPY neurons. J Neurosci. 2009;29:4622–39.
Lee K, Dixon AK, Gonzalez I, Stevens EB, McNulty S, Oles R, et al. Bombesin-like peptides depolarize rat hippocampal interneurones through interaction with subtype 2 bombesin receptors. J Pysiol. 1999;518:791–802.
Wada E, Way J, Lebacq-Verheyden AM, Battey JF. Neuromedin B and gastrin-releasing peptide mRNAs are differentially distributed in the rat nervous system. J Neurosci. 1990;10:2917–30.
Sun YG, Chen ZF. A gastrin-releasing peptide receptor mediates the itch sensation in the spinal cord. Nature 2007;448:700–3.
Li P, Janczewski WA, Yackle K, Kam K, Pagliardini S, Krasnow MA, et al. The peptidergic control circuit for sighing. Nature 2016;530:293–7.
Shumyatsky GP, Tsvetkov E, Malleret G, Vronskaya S, Hatton M, Hampton L, et al. Identification of a signaling network in lateral nucleus of amygdala important for inhibiting memory specifically related to learned fear. Cell 2002;111:905–18.
Merali Z, McIntosh J, Kent P, Michaud D, Anisman H. Aversive and appetitive events evoke the release of corticotropin-releasing hormone and bombesin-like peptides at the central nucleus of the amygdala. J Neurosci. 1998;18:4758–66.
Merali Z, Anisman H, James JS, Kent P, Schulkin J. Effects of corticosterone on corticotrophin-releasing hormone and gastrin-releasing peptide release in response to an aversive stimulus in two regions of the forebrain (central nucleus of the amygdala and prefrontal cortex). Eur J Neurosci. 2008;28:165–72.
Merali Z, Hayley S, Kent P, McIntosh J, Bedard T, Anisman H. Impact of repeated stressor exposure on the release of corticotropin-releasing hormone, arginine-vasopressin and bombesin-like peptides at the anterior pituitary. Behav Brain Res. 2009;198:105–12.
Krettek JE, Price JL. A description of the amygdaloid complex in the rat and cat with observations on intra-amygdaloid axonal connections. J Comp Neurol. 1978;178:255–80.
Smith Y, Pare D. Intra-amygdaloid projections of the lateral nucleus in the cat: PHA-L anterograde labeling combined with postembedding GABA and glutamate immunocytochemistry. J Comp Neurol. 1994;342:232–48.
Pitkanen A, Savander V, LeDoux JE. Organization of intra-amygdaloid circuitries in the rat: an emerging framework for understanding functions of the amygdala. Trends Neurosci. 1997;20:517–23.
Pare D, Duvarci S. Amygdala microcircuits mediating fear expression and extinction. Curr Opin Neurobiol. 2012;22:717–23.
Jolkkonen E, Pikkarainen M, Kemppainen S, Pitkanen A. Interconnectivity between the amygdaloid complex and the amygdalostriatal transition area: a PHA-L study in rat. J Comp Neurol. 2001;431:39–58.
Wang C, Kang-Park MH, Wilson WA, Moore SD. Properties of the pathways from the lateral amygdal nucleus to basolateral nucleus and amygdalostriatal transition area. J Neurophysiol. 2002;87:2593–601.
Leitermann RJ, Rostkowski AB, Urban JH. Neuropeptide Y input to the rat basolateral amygdala complex and modulation by conditioned fear. J Comp Neurol. 2016;524:2418–39.
Mikuni T, Uesaka N, Okuno H, Hirai H, Deisseroth K, Bito H, et al. Arc/Arg3.1 is a postsynaptic mediator of activity-dependent synapse elimination in the developing cerebellum. Neuron. 2013;78:1024–35.
Ohnishi T, Kiyama Y, Arima-Yoshida F, Kadota M, Ichikawa T, Yamada K, et al. Cooperation of LIM domain-binding 2 with EGR transcription factors to regulate gene expression networks relevant to mental disorders. EMBO Mol Med. 2021;13:e12574.
Susaki EA, Tainaka K, Perrin D, Kishino F, Tawara T, Watanabe TM, et al. Whole-brain imaging with single-cell resolution using chemical cocktails and computational analysis. Cell 2014;157:726–39.
Carpenter AE, Jones TR, Lamprecht MR, Clarke C, Kang IH, Friman O, et al. CellProfiler: image analysis software for identifying and quantifying cell phenotypes. Genome Biol. 2006;7:R100.
Kiyama Y, Manabe T, Sakimura K, Kawakami F, Mori H, Mishina M. Increased thresholds for long-term potentiation and contextual learning in mice lacking the NMDA-type glutamate receptor ε1 subunit. J Neurosci. 1998;18:6704–12. 44
Erb S, Funk D, Le AD. Cocaine pre-exposure enhances CRF-induced expression of c-fos mRNA in the central nucleus of the amygdala: an effect that parallels the effects of cocaine pre-exposure on CRF-induced locomotor activity. Neurosci Lett. 2005;383:209–14.
Felix-Ortiz AC, Beyeler A, Seo C, Leppla CA, Wildes CP, Tye KM. BLA to vHPC inputs modulate anxiety-related behaviors. Neuron 2013;79:658–64.
Walker DL, Davis M. The role of amygdala glutamate receptors in fear learning, fear-potentiated startle, and extinction. Pharm Biochem Behav. 2002;71:379–92.
Watabe AM, Ochiai T, Nagase M, Takahashi Y, Sato M, Kato F. Synaptic potentiation in the nociceptive amygdala following fear learning in mice. Mol Brain. 2013;6:11.
Neugebauer V. Amygdala pain mechanisms. Handb Exp Pharm. 2015;227:261–84.
Bellani M, Baiano M, Brambilla P. Brain anatomy of major depression II. Focus on amygdala. Epidemiol Psychiatr Sci. 2011;20:33–6.
Hughes RN. The value of spontaneous alternation behavior (SAB) as a test of retention in pharmacological investigations of memory. Neurosci Biobehav Rev. 2004;28:497–505.
Nakazawa T, Hashimoto R, Sakoori K, Sugaya Y, Tanimura A, Hashimotodani Y, et al. Emerging roles of ARHGAP33 in intracellular trafficking of TrkB and pathophysiology of neuropsychiatric disorders. Nat Commun. 2016;7:10594.
Kim JJ, DeCola JP, Landeira-Fernandez J, Fanselow MS. N-methyl-D-aspartate receptor antagonist APV blocks acquisition but not expression of fear conditioning. Behav Neurosci. 1991;105:126–33.
Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–85.
Roesler R, Kent P, Luft T, Schwartsmann G, Merali Z. Gastrin-releasing peptide receptor signaling in the integration of stress and memory. Neurobiol Learn Mem. 2014;112:44–52.
Murkar A, Kent P, Cayer C, James J, Merali Z. Gastrin-releasing peptide attenuates fear memory reconsolidation. Behav Brain Res. 2018;347:255–62.
Ehrlich I, Humeau Y, Grenier F, Ciocchi S, Herry C, Lüthi A. Amygdala inhibitory circuits and the control of fear memory. Neuron 2009;62:757–71.
Garrido MM, Martin S, Ambrosio E, Fuentes JA, Manzanares J. Role of corticotropin-releasing hormone in gastrin-releasing peptide-mediated regulation of corticotropin and corticosterone secretion in male rats. Neuroendocrinology 1998;68:116–22.
Roesler R, Lessa D, Venturella R, Vianna MR, Luft T, Henriques JA, et al. Bombesin/gastrin-releasing peptide receptors in the basolateral amygdala regulate memory consolidation. Eur J Neurosci. 2004;19:1041–45.
Mountney C, Anisman H, Merali Z. Effects of gastrin-releasing peptide agonist and antagonist administered to the basolateral nucleus of the amygdala on conditioned fear in the rat. Psychopharmacol (Berl). 2008;200:51–8.
Mountney C, Sillberg V, Kent P, Anisman H, Merali Z. The role of gastrin-releasing peptide on conditioned fear: differential cortical and amygdaloid responses in the rat. Psychopharmacol (Berl). 2006;189:287–96.
Morishita Y, Fuentes I, Favate J, Zushida K, Nishi A, Hevi C et al. The gastrin-releasing peptide regulates stress-enhanced fear and dopamine signaling. BioRxiv (2020) https://doi.org/10.1101/2020.12.31.424996.
Cordero M, MerinoJJ, Sandi C. Correlational relationship between shock intensity and corticosterone secretion on the establishment and subsequent expression of contextual fear conditioning. Behav Neurosci. 1998;112:885–91.
Marchand AR, Barbelivien A, Seillier A, Herbeaux K, Sarrieau A, Majchrzak M, et al. Contribution of corticosterone to cued versus contextual fear in rats. Behav Brain Res. 2007;183:101–10.
Chau LS, Prakapenka A, Fleming SA, Davis AS, Galvez R. Elevated Arc/Arg 3.1 protein expression in the basolateral amygdala following auditory trace-cued fear conditioning. Neurobiol Learn Mem. 2013;106:127–33.
Ploski JE, Pierre VJ, Smucny J, Park K, Monsey MS, Overeem KA, et al. The activity-regulated cytoskeletal-associated protein (Arc/Arg3.1) is required for memory consolidation of pavlovian fear conditioning in the lateral amygdala. J Neurosci. 2008;28:12383–95.
Duvarci S, Pare D. Amygdala microcircuits controlling learned fear. Neuron 2014;82:966–80.
Herry C, Ciocchi S, Senn V, Demmou L, Muller C, Luthi A. Switching on and off fear by distinct neuronal circuits. Nature 2008;454:600–6.
Amano T, Duvarci S, Popa D, Pare D. The fear circuit revisited: contributions of the basal amygdala nuclei to conditioned fear. J Neurosci. 2011;31:15481–89.
Calandreau L, Desmedt A, Decorte L, Jaffard R. A different recruitment of the lateral and basolateral amygdala promotes contextual or elemental conditioned association in Pavlovian fear conditioning. Learn Mem. 2005;12:383–8.
Tang S, Li H, Lu L, Wang Y, Zhang L, Hu X, et al. Anomalous functional connectivity of amygdala subregional networks in major depressive disorder. Depress Anxiety. 2019;36:712–22.
Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci. 2011;15:85–93.
Fu CH, Williams SC, Brammer MJ, Suckling J, Kim J, Cleare AJ, et al. Neural responses to happy facial expressions in major depression following antidepressant treatment. Am J Psychiatry. 2007;164:599–607.
McDonald AJ, Zaric V. Extrinsic origins of the somatostatin and neuropeptide Y innervation of the rat basolateral amygdala. Neuroscience 2015;294:82–100.
Wolff SB, Grundemann J, Tovote P, Krabbe S, Jacobson GA, Muller C, et al. Amygdala interneuron subtypes control fear learning through disinhibition. Nature 2014;509:453–8.
DeVylder JE, Koyanagi A, Unick J, Oh H, Nam B, Stickley A. Stress Sensitivity and Psychotic Experiences in 39 Low- and Middle-Income Countries. Schizophr Bull. 2016;42:1353–62.
Acknowledgements
We thank S. Sato, N. Numata, M. Nagai, Y. Watanabe, and R. Shiojiri for maintaining mouse colonies and R.S. Suzuki for technical assistances. This work was supported by JSPS KAKENHI Grant Number 21200006, 15K01848 and 19K07799 to YK; 18H05127, 19H03328 and 20H05068 to HO; 15H02358, 17K19442, and 17H06312 to HB; 18100003, 23220008, 25116505, 15K14309, 19H03321 and 19H04876 to TM.
Author information
Authors and Affiliations
Contributions
YK and TM conceived the idea and coordinated the study. IO, TI, HI, and NY generated GRP-KO mice. FG and YK performed the behavioral experiments. HO and HB generated Arc-Venus reporter transgenic mouse. FG, YK, and IO performed histological analysis. MA and TK provided support for paper writing. FG, YK, and TM participated in paper writing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Goto, F., Kiyama, Y., Ogawa, I. et al. Gastrin-releasing peptide regulates fear learning under stressed conditions via activation of the amygdalostriatal transition area. Mol Psychiatry 27, 1694–1703 (2022). https://doi.org/10.1038/s41380-021-01408-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-021-01408-3