Abstract
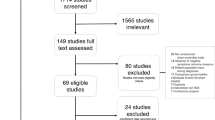
There is growing evidence that placebo effects can meaningfully modulate the brain. However, there has been little consideration of whether these changes may overlap with regions/circuits targeted by depression treatments and what the implications of this overlap would be on measuring efficacy in placebo-controlled clinical trials. In this systematic review and meta-analysis, we searched PubMed/Medline and Google Scholar for functional MRI and PET neuroimaging studies of placebo effects. Studies recruiting both healthy subjects and patient populations were included. Neuroimaging coordinates were extracted and included for Activation Likelihood Estimation (ALE) meta-analysis. We then searched for interventional studies of transcranial magnetic stimulation (TMS) and deep brain stimulation (DBS) for depression and extracted target coordinates for comparative spatial analysis with the placebo effects maps. Of 1169 articles identified, 34 neuroimaging studies of placebo effects were included. There were three significant clusters of activation: left dorsolateral prefrontal cortex (DLPFC) (x = −41, y = 16, z = 34), left sub-genual anterior cingulate cortex (sgACC)/ventral striatum (x = −8, y = 18, z = −15) and the right rostral anterior cingulate cortex (rACC) (x = 4, y = 42, z = 10). There were two significant deactivation clusters: right basal ganglia (x = 20, y = 2, z = 7) and right dorsal anterior cingulate cortex (dACC) (x = 1, y = −5, z = 45). TMS and DBS targets for depression treatment overlapped with the left DLPFC cluster and sgACC cluster, respectively. Our findings identify a common set of brain regions implicated in placebo effects across healthy individuals and patient populations, and provide evidence that these regions overlap with depression treatment targets. We model the statistical impacts of this overlap and demonstrate critical implications on measurements of clinical trial efficacy for this field.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Wager TD, Atlas LY. The neuroscience of placebo effects: connecting context, learning and health. Nat Rev Neurosci. 2015;16:403–18. https://doi.org/10.1038/nrn3976.
Kaptchuk TJ, Hemond CC, Miller FG. Placebos in chronic pain: evidence, theory, ethics, and use in clinical practice. Bmj Published online July. 2020;20:m1668 https://doi.org/10.1136/bmj.m1668.
Colloca L, Barsky AJ. Placebo and nocebo effects. N Engl J Med. 2020;382:554–61.
Amanzio M, Benedetti F, Porro CA, Palermo S, Cauda F Activation likelihood estimation meta‐analysis of brain correlates of placebo analgesia in human experimental pain. Hum Brain Mapp. 2013;34.
Kaptchuk TJ, Miller FG Placebo effects in medicine. N Engl J Med. 2015;373.
Marchant J. Strong placebo response thwarts painkiller trials. Potential pain treatments are struggling to prove their worth over a rising placebo effect seen in US trials. Nature 2015;6:6.
Weimer K, Colloca L, Enck P. Placebo effects in psychiatry: mediators and moderators. Lancet Psychiatry. 2015;2:246–57. https://doi.org/10.1016/S2215-0366(14)00092-3.
Walsh BT, Seidman SN, Sysko R, Gould M. Placebo response in studies of major depression: variable, substantial, and growing. JAMA. 2002;287:1840–1847. https://doi.org/10.1001/jama.287.14.1840.
Mayberg HS, Silva JA, Brannan SK, Tekell JL, Mahurin RK, McGinnis S, et al. The functional neuroanatomy of the placebo effect. Am J Psychiatry. 2002;159:728–37. https://doi.org/10.1176/appi.ajp.159.5.728.
Kirsch I The Placebo effect in the treatment of depression and anxiety. Front Psychiatry. 2019;10.
Burke MJ, Kaptchuk TJ, Pascual-Leone A Challenges of differential placebo effects in contemporary medicine: the example of brain stimulation. Ann Neurol. 2019;85.
Yesavage JA, Fairchild JK, Mi Z, Biswas K, Davis-Karim A, Phibbs CS et al. Effect of repetitive transcranial magnetic stimulation on treatment-resistant major depression in US veterans: a randomized clinical trial. JAMA Psychiatry. 2018;75.
Razza LB, Moffa AH, Moreno ML, Carvalho AF, Padberg F, Fregni F, et al. A systematic review and meta-analysis on placebo response to repetitive transcranial magnetic stimulation for depression trials. Prog Neuropsychopharmacol Biol Psychiatry. 2018;81:105–13.
Eickhoff SB, Bzdok D, Laird AR, Kurth F, Fox PT. Activation Likelihood Estimation meta-analysis revisited. Neuroimage. 2012;59:2349–61. https://doi.org/10.1016/j.neuroimage.2011.09.017
Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA. Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. NeuroImage. 2002;16:765–80. https://doi.org/10.1006/nimg.2002.1131
Marcus DS, Harwell J, Olsen T, Hodge M, Glasser M, Prior F et al. Informatics and Data mining tools and strategies for the human connectome project. Front Neuroinformatics. 2011;5. https://doi.org/10.3389/fninf.2011.00004
Enck P, Horing B, Broelz E, Weimer K. Knowledge gaps in placebo research: with special reference to neurobiology. Int Rev Neurobiol. 2018;139:85–106. https://doi.org/10.1016/bs.irn.2018.07.018
Ashar YK, Chang LJ, Wager TD. Brain mechanisms of the placebo effect: an affective appraisal account. Annu Rev Clin Psychol. 2017;13:73–98. https://doi.org/10.1146/annurev-clinpsy-021815-093015
Peciña M, Bohnert ASB, Sikora M, Avery ET, Langenecker SA, Mickey BJ, et al. Association Between placebo-activated neural systems and antidepressant responses: neurochemistry of placebo effects in major depression. JAMA Psychiatry. 2015;72:1087 https://doi.org/10.1001/jamapsychiatry.2015.1335
Burke MJ, Fried PJ, Pascual-Leone A. Transcranial magnetic stimulation: neurophysiological and clinical applications. Handbook of clinical neurology. Elsevier; 2019. p. 73–92.
Rossi S, Hallett M, Rossini PM, Pascual-Leone A. Safety of TMS Consensus Group. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol J Int Fed Clin Neurophysiol. 2009;120:2008–39. https://doi.org/10.1016/j.clinph.2009.08.016
Brunoni AR, Chaimani A, Moffa AH, Razza LB, Gattaz WF, Daskalakis ZJ, et al. Repetitive transcranial magnetic stimulation for the acute treatment of major depressive episodes: a systematic review with network meta-analysis. JAMA Psychiatry. 2017;74:143–52. https://doi.org/10.1001/jamapsychiatry.2016.3644
Fox MD, Buckner RL, White MP, Greicius MD, Pascual-Leone A. Efficacy of transcranial magnetic stimulation targets for depression is related to intrinsic functional connectivity with the subgenual cingulate. Biol Psychiatry. 2012;72:595–603. https://doi.org/10.1016/j.biopsych.2012.04.028
Siddiqi SH, Taylor SF, Cooke D, Pascual-Leone A, George MS, Fox MD. Distinct symptom-specific treatment targets for circuit-based neuromodulation. Am J Psychiatry. 2020;177:435–46. https://doi.org/10.1176/appi.ajp.2019.19090915
Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–60. https://doi.org/10.1016/j.neuron.2005.02.014
Weigand A, Horn A, Caballero R, Cooke D, Stern AP, Taylor SF, et al. Prospective validation that subgenual connectivity predicts antidepressant efficacy of transcranial magnetic stimulation sites. Biol Psychiatry. 2018;84:28–37.
Wu GR, Wang X, Baeken C Baseline functional connectivity may predict placebo responses to accelerated rTMS treatment in major depression. Hum Brain Mapp. 2020;41.
O’Reardon JP, Solvason HB, Janicak PG, Sampson S, Isenberg KE, Nahas Z, et al. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol Psychiatry. 2007;62:1208–16. https://doi.org/10.1016/j.biopsych.2007.01.018
Valkonen-Korhonen M, Leinola H, Könönen M, Niskanen E, Purhonen M, Pakarinen M et al. Bifrontal active and sham rTMS in treatment-resistant unipolar major depression. Nord J Psychiatry. 2018;72.
McCambridge J, Witton J, Elbourne DR. Systematic review of the Hawthorne effect: new concepts are needed to study research participation effects. J Clin Epidemiol. 2014;67:267–77. https://doi.org/10.1016/j.jclinepi.2013.08.015
Shrout PE, Stadler G, Lane SP, McClure MJ, Jackson GL, Clavél FD, et al. Initial elevation bias in subjective reports. Proc Natl Acad Sci. 2018;115:E15–E23. https://doi.org/10.1073/pnas.1712277115
Enck P, Bingel U, Schedlowski M, Rief W. The placebo response in medicine: minimize, maximize or personalize? Nat Rev Drug Disco. 2013;12:191–204. https://doi.org/10.1038/nrd3923
Mestre TA, Lang AE, Okun MS Factors influencing the outcome of deep brain stimulation: placebo, nocebo, lessebo, and lesion effects. Mov Disord. 2016;31.
Holtzheimer PE, Husain MM, Lisanby SH, Taylor SF, Whitworth LA, McClintock S et al. Subcallosal cingulate deep brain stimulation for treatment-resistant depression: a multisite, randomised, sham-controlled trial. Lancet Psychiatry. 2017;4.
Li C-T, Chen M-H, Juan C-H, Huang HH, Chen LF, Hsieh JC, et al. Efficacy of prefrontal theta-burst stimulation in refractory depression: a randomized sham-controlled study. Brain. 2014;137:2088–98. https://doi.org/10.1093/brain/awu109
Brunoni AR, Lopes M, Kaptchuk TJ, Fregni F Placebo response of non-pharmacological and pharmacological trials in major depression: a systematic review and meta-analysis. Hashimoto K, ed. PLoS ONE. 2009;4:e4824. https://doi.org/10.1371/journal.pone.0004824
Lam RW, Chan P, Wilkins-Ho M, Yatham LN. Repetitive transcranial magnetic stimulation for treatment-resistant depression: a systematic review and metaanalysis. Can J Psychiatry. 2008;53:621–31. https://doi.org/10.1177/070674370805300909
Herrington TM, Cheng JJ, Eskandar EN Mechanisms of deep brain stimulation. J Neurophysiol. 2016;115.
Enck P, Zipfel S. Placebo effects in psychotherapy: a framework. Front Psychiatry. 2019;10:456. https://doi.org/10.3389/fpsyt.2019.00456
Erekson DM, Lambert MJ, Eggett DL The relationship between session frequency and psychotherapy outcome in a naturalistic setting. J Consult Clin Psychol. 83:1097–107.
Leuchter AF, Hunter AM, Tartter M, Cook IA. Role of pill-taking, expectation and therapeutic alliance in the placebo response in clinical trials for major depression. Br J Psychiatry. 2014;205:443–9. https://doi.org/10.1192/bjp.bp.113.140343
Kaptchuk TJ, Kelley JM, Conboy LA, Davis RB, Kerr CE, Jacobson EE, et al. Components of placebo effect: randomised controlled trial in patients with irritable bowel syndrome. BMJ. 2008;336:999–1003. https://doi.org/10.1136/bmj.39524.439618.25
Lefaucheur J-P, Antal A, Ayache SS, Benninger DH, Brunelin J, Cogiamanian F, et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin Neurophysiol J Int Fed Clin Neurophysiol. 2017;128:56–92. https://doi.org/10.1016/j.clinph.2016.10.087
Schambra H, Bikson M, Wager T, DosSantos M, DaSilva A. It’s all in your head: reinforcing the placebo response with tDCS. Brain Stimul. 2014;7:623–624. https://doi.org/10.1016/j.brs.2014.04.002
Maeda F, Keenan JP, Tormos JM, Topka H, Pascual-Leone A. Modulation of corticospinal excitability by repetitive transcranial magnetic stimulation. Clin Neurophysiol Off J Int Fed. Clin Neurophysiol. 2000;111:800–805. https://doi.org/10.1016/s1388-2457(99)00323-5
Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci. 2011;15:483–506. https://doi.org/10.1016/j.tics.2011.08.003
Krummenacher P, Candia V, Folkers G, Schedlowski M, Schönbächler G. Prefrontal cortex modulates placebo analgesia. Pain. 2010;148:368–74. https://doi.org/10.1016/j.pain.2009.09.033
Benedetti F, Carlino E, Pollo A Hidden administration of drugs. Clin Pharmacol Ther. 2011;90.
Acknowledgements
This study was supported by funding from the Liu Fu Yu Charity Foundation and Sidney R. Baer, Jr. Foundation.
Author information
Authors and Affiliations
Contributions
Concept and Design: MB, ES, APL, TJK, MDF. Data collection and processing: SR, RG, LM. Statistical analysis: SR, LM, ES. Drafting of the manuscript: MB, SR, ES. Critical revision of the manuscript: MDF, APL, TJK.
Corresponding authors
Ethics declarations
Competing interests
MJB has nothing to disclose. ES has nothing to disclose. SR has nothing to disclose. LM has nothing to disclose. RG has nothing to disclose. APL has nothing to disclose. TK has nothing to disclose. MDF has intellectual property on using connectivity imaging to guide brain stimulation but receives no royalties.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Burke, M.J., Romanella, S.M., Mencarelli, L. et al. Placebo effects and neuromodulation for depression: a meta-analysis and evaluation of shared mechanisms. Mol Psychiatry 27, 1658–1666 (2022). https://doi.org/10.1038/s41380-021-01397-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-021-01397-3
This article is cited by
-
Connectivity-guided intermittent theta burst versus repetitive transcranial magnetic stimulation for treatment-resistant depression: a randomized controlled trial
Nature Medicine (2024)
-
Study protocol: effects of treatment expectation toward repetitive transcranial magnetic stimulation (rTMS) in major depressive disorder—a randomized controlled clinical trial
Trials (2023)
-
Optimizing transcranial magnetic stimulation for spaceflight applications
npj Microgravity (2023)
-
Growing placebo response in TMS treatment for depression: a meta-analysis of 27-year randomized sham-controlled trials
Nature Mental Health (2023)
-
Expectancy in placebo-controlled trials of psychedelics: if so, so what?
Psychopharmacology (2022)