Abstract
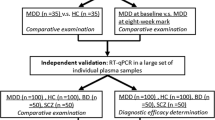
Previous work has demonstrated that microRNAs (miRNAs) change as a function of antidepressant treatment (ADT) response. However, it is unclear how representative these peripherally detected miRNA changes are to those occurring in the brain. This study aimed to use peripherally extracted neuron-derived extracellular vesicles (NDEV) to circumvent these limitations and investigate neuronal miRNA changes associated with antidepressant response. Samples were collected at two time points (baseline and after 8 weeks of follow-up) from depressed patients who responded (N = 20) and did not respond (N = 20) to escitalopram treatment, as well as controls (N = 20). Total extracellular vesicles (EVs) were extracted from plasma, and then further enriched for NDEV by immunoprecipitation with L1CAM. EVs and NDEVs were characterized, and NDEV miRNA cargo was extracted and sequenced. Subsequently, studies in cell lines and postmortem tissue were conducted. Characterization of NDEVs revealed that they were smaller than other EVs isolated from plasma (p < 0.0001), had brain-specific neuronal markers, and contained miRNAs enriched for brain functions (p < 0.0001) Furthermore, NDEVs from depressed patients were smaller than controls (p < 0.05), and NDEV size increased with ADT response (p < 0.01). Finally, changes in NDEV cargo, specifically changes in miR-21-5p, miR-30d-5p, and miR-486-5p together (p < 0.01), were associated with ADT response. Targets of these three miRNAs were altered in brain tissue from depressed individuals (p < 0.05). Together, this study indicates that changes in peripherally isolated NDEV can act as both a clinically accessible and informative biomarker of ADT response specifically through size and cargo.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
WHO. Depression. 2020. Available from: https://www.who.int/news-room/fact-sheets/detail/depression.
Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR* D report. Am J Psychiatry. 2006;163:1905–17.
Belzeaux R, Lin R, Turecki G. Potential use of MicroRNA for monitoring therapeutic response to antidepressants. CNS Drugs. 2017;31:253–62.
Lopez JP, Fiori LM, Cruceanu C, Lin R, Labonte B, Cates HM, et al. MicroRNAs 146a/b-5 and 425-3p and 24-3p are markers of antidepressant response and regulate MAPK/Wnt-system genes. Nat Commun. 2017;8:15497.
Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654.
Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29:341–5.
Saeedi S, Israel S, Nagy C, Turecki G. The emerging role of exosomes in mental disorders. Transl Psychiatry. 2019;9:122.
Gallo A, Tandon M, Alevizos I, Illei GG. The majority of microRNAs detectable in serum and saliva is concentrated in exosomes. PLoS ONE. 2012;7:e30679.
Taylor DD, Gercel-Taylor C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol Oncol. 2008;110:13–21.
Goetzl EJ, Mustapic M, Kapogiannis D, Eitan E, Lobach IV, Goetzl L, et al. Cargo proteins of plasma astrocyte-derived exosomes in Alzheimer’s disease. FASEB J. 2016;30:3853–9.
Mustapic M, Eitan E, Werner JK, Berkowitz ST, Lazaropoulos MP, Tran J, et al. Plasma extracellular vesicles enriched for neuronal origin: a potential window into brain pathologic processes. Front Neurosci. 2017;11:278.
Cha DJ, Mengel D, Mustapic M, Liu W, Selkoe DJ, Kapogiannis D, et al. miR-212 and miR-132 are downregulated in neurally derived plasma exosomes of Alzheimer’s patients. Front Neurosci. 2019;13:1208.
Goetzl EJ, Abner EL, Jicha GA, Kapogiannis D, Schwartz JB. Declining levels of functionally specialized synaptic proteins in plasma neuronal exosomes with progression of Alzheimer’s disease. FASEB J. 2018;32:888–93.
Sun B, Dalvi P, Abadjian L, Tang N, Pulliam L. Blood neuron-derived exosomes as biomarkers of cognitive impairment in HIV. AIDS 2017;31:F9–17.
Nasca C, Dobbin J, Bigio B, Watson K, de Angelis P, Kautz M, et al. Insulin receptor substrate in brain-enriched exosomes in subjects with major depression: on the path of creation of biosignatures of central insulin resistance. Mol Psychiatry. 2020. https://doi.org/10.1038/s41380-020-0804-7. Epub ahead of print. PMID: 32536688.
Mansur RB, Delgado-Peraza F, Subramaniapillai M, Lee Y, Iacobucci M, Rodrigues N, et al. Extracellular vesicle biomarkers reveal inhibition of neuroinflammation by infliximab in association with antidepressant response in adults with bipolar depression. Cells 2020;9:895.
Jollant F, Perreira F, Fiori LM, Richard-Devantoy S, Lutz PE, Belzeaux R, et al. Neural and molecular correlates of psychological pain during major depression, and its link with suicidal ideas. Prog Neuro-Psychopharmacol Biol Psychiatry. 2020;100:109909.
Rikkert LG, Nieuwland R, Terstappen LWMM, Coumans FAW. Quality of extracellular vesicle images by transmission electron microscopy is operator and protocol dependent. J Extracell Vesicles. 2019;8:1555419.
Stoeck A, Keller S, Riedle S, Sanderson MP, Runz S, Le Naour F, et al. A role for exosomes in the constitutive and stimulus-induced ectodomain cleavage of L1 and CD44. Biochem J. 2006;393:609–18.
Ogawa Y, Taketomi Y, Murakami M, Tsujimoto M, Yanoshita R. Small RNA transcriptomes of two types of exosomes in human whole saliva determined by next generation sequencing. Biol Pharm Bull. 2013;36:66–75.
Bailey TL, Boden M, Buske FA, Frith M, Grant CE, Clementi L. et al. MEME SUITE: tools for motif discovery and searching. Nucleic Acids Res. 2009;37 suppl_2:W202–8.
Zerbino DR, Achuthan P, Akanni W, Amode MR, Barrell D, Bhai J, et al. Ensembl 2018. Nucleic Acids Res. 2017;46:D754–61.
Kuleshov MV, Jones MR, Rouillard AD, Fernandez NF, Duan Q, Wang Z, et al. Enrichr: a comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 2016;44:W90–7.
Lachmann A, Torre D, Keenan AB, Jagodnik KM, Lee HJ, Wang L, et al. Massive mining of publicly available RNA-seq data from human and mouse. Nat Commun. 2018;9:1366.
Bandari SK, Purushothaman A, Ramani VC, Brinkley GJ, Chandrashekar DS, Varambally S, et al. Chemotherapy induces secretion of exosomes loaded with heparanase that degrades extracellular matrix and impacts tumor and host cell behavior. Matrix Biol. 2018;65:104–18.
Hessvik NP, Llorente A. Current knowledge on exosome biogenesis and release. Cell Mol Life Sci. 2018;75:193–208.
Colombo M, Moita C, van Niel G, Kowal J, Vigneron J, Benaroch P, et al. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J Cell Sci. 2013;126:5553–65.
Rodgers AB, Morgan CP, Bronson SL, Revello S, Bale TL. Paternal stress exposure alters sperm microRNA content and reprograms offspring HPA stress axis regulation. J Neurosci. 2013;33:9003–12.
Kim HK, Tyryshkin K, Elmi N, Dharsee M, Evans KR, Good J, et al. Plasma microRNA expression levels and their targeted pathways in patients with major depressive disorder who are responsive to duloxetine treatment. J Psychiatr Res. 2019;110:38–44.
Maness PF, Schachner M. Neural recognition molecules of the immunoglobulin superfamily: signaling transducers of axon guidance and neuronal migration. Nat Neurosci. 2007;10:19–26.
Wingo TS, Yang J, Fan W, Min Canon S, Gerasimov ES, Lori A, et al. Brain microRNAs associated with late-life depressive symptoms are also associated with cognitive trajectory and dementia. npj Genom Med. 2020;5:6.
Dwivedi Y. Emerging role of microRNAs in major depressive disorder: diagnosis and therapeutic implications. Dialogues Clin Neurosci. 2014;16:43–61.
Pariante CM, Lightman SL. The HPA axis in major depression: classical theories and new developments. Trends Neurosci. 2008;31:464–8.
Uhlén M, Fagerberg L, Hallström BM, Lindskog C, Oksvold P, Mardinoglu A, et al. Proteomics. Tissue-based map of the human proteome. Science. 2015;347:1260419.
Tu C, Du T, Shao C, Liu Z, Li L, Shen Y. Evaluating the potential of housekeeping genes, rRNAs, snRNAs, microRNAs and circRNAs as reference genes for the estimation of PMI. Forensic Sci, Med, Pathol. 2018;14:194–201.
Nagy C, Maheu M, Lopez JP, Vaillancourt K, Cruceanu C, Gross JA, et al. Effects of postmortem interval on biomolecule integrity in the brain. J Neuropathol Exp Neurol. 2015;74:459–69.
Acknowledgements
GT holds a Canada Research Chair (Tier 1) and is supported by grants from the Canadian Institute of Health Research (CIHR) (FDN148374 and EPN161427 (ERA-NET ERA PerMed)), and by the Fonds de recherche du Québec—Santé (FRQS) through the Quebec Network on Suicide, Mood Disorders, and Related Disorders. CAN-BIND is an Integrated Discovery Program carried out in partnership with, and financial support from, the Ontario Brain Institute, an independent non-profit corporation, funded partially by the Ontario government. The opinions, results and conclusions are those of the authors and no endorsement by the Ontario Brain Institute is intended or should be inferred. Funding support was also provided by a grant from the Ontario Research Fund: Research Excellence (RE-08-027) SD. The Douglas-Bell Canada Brain Bank is supported in part by funding from the Canada First Research Excellence Fund, awarded to McGill University for the Healthy Brains for Healthy Lives initiative, and from the Fonds de recherche du Québec—Santé (FRQS) through the Quebec Network on Suicide, Mood Disorders and Related Disorders. SS is funded by the Canadian Institute of Health Research (CIHR).
Author information
Authors and Affiliations
Contributions
Conceptualization: SS, CN, and GT. Methodology: SS, CN, MW, and J-FT. Formal analysis: SS and J-FT. Investigation: SS, CN, MW, PI, LMF, and JY. Writing—original draft: SS, CN, and GT. Writing—review and editing: J-FT, LMF, PI, SR, SHK, JAF, and NM. Resources: SR, SHK, JAF, NM, and GT. Funding acquisition: GT, SR, SHK, JAF, NM.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Saeedi, S., Nagy, C., Ibrahim, P. et al. Neuron-derived extracellular vesicles enriched from plasma show altered size and miRNA cargo as a function of antidepressant drug response. Mol Psychiatry 26, 7417–7424 (2021). https://doi.org/10.1038/s41380-021-01255-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-021-01255-2
This article is cited by
-
Adipose-derived extracellular vesicles – a novel cross-talk mechanism in insulin resistance, non-alcoholic fatty liver disease, and polycystic ovary syndrome
Endocrine (2024)
-
miR-9-5p is Downregulated in Serum Extracellular Vesicles of Patients Treated with Biperiden After Traumatic Brain Injury
Molecular Neurobiology (2024)
-
A review of the regulatory mechanisms of extracellular vesicles-mediated intercellular communication
Cell Communication and Signaling (2023)
-
The role of Extracellular Genomic Materials (EGMs) in psychiatric disorders
Translational Psychiatry (2023)
-
MicroRNA therapeutic targets in neonatal hypoxic–ischemic brain injury: a narrative review
Pediatric Research (2023)