Abstract
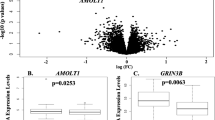
Posttraumatic stress disorder (PTSD) is a debilitating syndrome with substantial morbidity and mortality that occurs in the aftermath of trauma. Symptoms of major depressive disorder (MDD) are also a frequent consequence of trauma exposure. Identifying novel risk markers in the immediate aftermath of trauma is a critical step for the identification of novel biological targets to understand mechanisms of pathophysiology and prevention, as well as the determination of patients most at risk who may benefit from immediate intervention. Our study utilizes a novel approach to computationally integrate blood-based transcriptomics, genomics, and interactomics to understand the development of risk vs. resilience in the months following trauma exposure. In a two-site longitudinal, observational prospective study, we assessed over 10,000 individuals and enrolled >700 subjects in the immediate aftermath of trauma (average 5.3 h post-trauma (range 0.5–12 h)) in the Grady Memorial Hospital (Atlanta) and Jackson Memorial Hospital (Miami) emergency departments. RNA expression data and 6-month follow-up data were available for 366 individuals, while genotype, transcriptome, and phenotype data were available for 297 patients. To maximize our power and understanding of genes and pathways that predict risk vs. resilience, we utilized a set-cover approach to capture fluctuations of gene expression of PTSD or depression-converting patients and non-converting trauma-exposed controls to find representative sets of disease-relevant dysregulated genes. We annotated such genes with their corresponding expression quantitative trait loci and applied a variant of a current flow algorithm to identify genes that potentially were causal for the observed dysregulation of disease genes involved in the development of depression and PTSD symptoms after trauma exposure. We obtained a final list of 11 driver causal genes related to MDD symptoms, 13 genes for PTSD symptoms, and 22 genes in PTSD and/or MDD. We observed that these individual or combined disorders shared ESR1, RUNX1, PPARA, and WWOX as driver causal genes, while other genes appeared to be causal driver in the PTSD only or MDD only cases. A number of these identified causal pathways have been previously implicated in the biology or genetics of PTSD and MDD, as well as in preclinical models of amygdala function and fear regulation. Our work provides a promising set of initial pathways that may underlie causal mechanisms in the development of PTSD or MDD in the aftermath of trauma.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Keane TM, Marx BP, Sloan DM. Post-traumatic stress disorder: definition, prevalence, and risk factors. In: LeDoux JE, Keane T, Shiromani P, editors. Post-traumatic stress disorder: basic science and clinical practice. Totowa, NJ: Humana Press; 2009, pp 1–19.
Rothbaum BO, Foa EB, Riggs DS, Murdock T, Walsh W. A prospective examination of post-traumatic stress disorder in rape victims. J Trauma Stress. 1992;5:455–75.
Stein MB, Kennedy C. Major depressive and post-traumatic stress disorder comorbidity in female victims of intimate partner violence. J Affect Disord. 2001;66:133–8.
Walter KH, Levine JA, Highfill-McRoy RM, Navarro M, Thomsen CJ. Prevalence of posttraumatic stress disorder and psychological comorbidities among U.S. Active Duty Service Members, 2006-2013. J Trauma Stress. 2018;31:837–44.
Hoppen TH, Morina N. The prevalence of PTSD and major depression in the global population of adult war survivors: a meta-analytically informed estimate in absolute numbers. Eur J Psychotraumatol. 2019;10:1578637.
Muscatelli S, Spurr H, OʼHara NN, OʼHara LM, Sprague SA, Slobogean GP. Prevalence of depression and posttraumatic stress disorder after acute orthopaedic trauma: a systematic review and meta-analysis. J Orthop Trauma. 2017;31:47–55.
Ravi M, Stevens JS, Michopoulos V. Neuroendocrine pathways underlying risk and resilience to PTSD in women. Front Neuroendocrinol. 2019;55:100790.
Ramikie TS, Ressler KJ. Mechanisms of sex differences in fear and posttraumatic stress disorder. Biol Psychiatry. 2018;83:876–85.
Brewin CR, Andrews B, Valentine JD. Meta-analysis of risk factors for posttraumatic stress disorder in trauma-exposed adults. J Consult Clin Psychol. 2000;68:748–66.
Tortella-Feliu M, Fullana MA, Pérez-Vigil A, Torres X, Chamorro J, Littarelli SA, et al. Risk factors for posttraumatic stress disorder: an umbrella review of systematic reviews and meta-analyses. Neurosci Biobehav Rev. 2019;107:154–65.
Sayed S, Iacoviello BM, Charney DS. Risk factors for the development of psychopathology following trauma. Curr Psychiatry Rep. 2015;17:612.
Binder EB, Bradley RG, Liu W, Epstein MP, Deveau TC, Mercer KB, et al. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. JAMA. 2008;299:1291–305.
McLaughlin KA, Koenen KC, Bromet EJ, Karam EG, Liu H, Petukhova M, et al. Childhood adversities and post-traumatic stress disorder: evidence for stress sensitisation in the World Mental Health Surveys. Br J Psychiatry. 2017;211:280–8.
Keane TM, Marshall AD, Taft CT. Posttraumatic stress disorder: etiology, epidemiology, and treatment outcome. Annu Rev Clin Psychol. 2006;2:161–97.
Platt J, Keyes KM, Koenen KC. Size of the social network versus quality of social support: which is more protective against PTSD? Soc Psychiatry Psychiatr Epidemiol. 2014;49:1279–86.
Logue MW, van Rooij SJH, Dennis EL, Davis SL, Hayes JP, Stevens JS, et al. Smaller hippocampal volume in posttraumatic stress disorder: a multisite ENIGMA-PGC study: subcortical volumetry results from posttraumatic stress disorder consortia. Biol Psychiatry. 2018;83:244–53.
Bremner JD, Vythilingam M, Vermetten E, Southwick SM, McGlashan T, Nazeer A, et al. MRI and PET study of deficits in hippocampal structure and function in women with childhood sexual abuse and posttraumatic stress disorder. Am J Psychiatry. 2003;160:924–32.
Afifi TO, Asmundson GJ, Taylor S, Jang KL. The role of genes and environment on trauma exposure and posttraumatic stress disorder symptoms: a review of twin studies. Clin Psychol Rev. 2010;30:101–12.
Koenen KC, Hitsman B, Lyons MJ, Niaura R, McCaffery J, Goldberg J, et al. A twin registry study of the relationship between posttraumatic stress disorder and nicotine dependence in men. Arch Gen Psychiatry. 2005;62:1258–65.
Koenen KC, Lyons MJ, Goldberg J, Simpson J, Williams WM, Toomey R, et al. A high risk twin study of combat-related PTSD comorbidity. Twin Res. 2003;6:218–26.
Stein MB, Jang KL, Taylor S, Vernon PA, Livesley WJ. Genetic and environmental influences on trauma exposure and posttraumatic stress disorder symptoms: a twin study. Am J Psychiatry. 2002;159:1675–81.
True WR, Rice J, Eisen SA, Heath AC, Goldberg J, Lyons MJ, et al. A twin study of genetic and environmental contributions to liability for posttraumatic stress symptoms. Arch Gen Psychiatry. 1993;50:257–64.
Xian H, Chantarujikapong SI, Scherrer JF, Eisen SA, Lyons MJ, Goldberg J, et al. Genetic and environmental influences on posttraumatic stress disorder, alcohol and drug dependence in twin pairs. Drug Alcohol Depend. 2000;61:95–102.
Cavalli G, Heard E. Advances in epigenetics link genetics to the environment and disease. Nature. 2019;571:489–99.
Dhaini HR, Daher Z. Genetic polymorphisms of PPAR genes and human cancers: evidence for gene-environment interactions. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2019;37:146–79.
Cust AE, Mishra K, Berwick M. Melanoma—role of the environment and genetics. Photochem Photobio Sci. 2018;17:1853–60.
Chang X, Dorajoo R, Sun Y, Han Y, Wang L, Khor CC, et al. Gene-diet interaction effects on BMI levels in the Singapore Chinese population. Nutr J. 2018;17:31.
Kaul N, Ali S. Genes, genetics, and environment in type 2 diabetes: implication in personalized medicine. DNA Cell Biol. 2016;35:1–12.
Hawn SE, Sheerin CM, Lind MJ, Hicks TA, Marraccini ME, Bountress K, et al. GxE effects of FKBP5 and traumatic life events on PTSD: A meta-analysis. J Affect Disord. 2019;243:455–62.
Smoller JW. The genetics of stress-related disorders: PTSD, depression, and anxiety disorders. Neuropsychopharmacology. 2016;41:297–319.
Rabl U, Meyer BM, Diers K, Bartova L, Berger A, Mandorfer D, et al. Additive gene-environment effects on hippocampal structure in healthy humans. J Neurosci. 2014;34:9917–26.
Broadaway KA, Duncan R, Conneely KN, Almli LM, Bradley B, Ressler KJ, et al. Kernel approach for modeling interaction effects in genetic association studies of complex quantitative traits. Genet Epidemiol. 2015;39:366–75.
Nievergelt CM, Maihofer AX, Klengel T, Atkinson EG, Chen CY, Choi KW, et al. International meta-analysis of PTSD genome-wide association studies identifies sex- and ancestry-specific genetic risk loci. Nat Commun. 2019;10:4558.
Gelernter J, Sun N, Polimanti R, Pietrzak R, Levey DF, Bryois J, et al. Genome-wide association study of post-traumatic stress disorder reexperiencing symptoms in >165,000 US veterans. Nat Neurosci. 2019;22:1394–401.
Segman RH, Shefi N, Goltser-Dubner T, Friedman N, Kaminski N, Shalev AY. Peripheral blood mononuclear cell gene expression profiles identify emergent post-traumatic stress disorder among trauma survivors. Mol Psychiatry. 2005;10:500–13. 425
Linnstaedt SD, Pan Y, Mauck MC, Sullivan J, Zhou CY, Jung L, et al. Evaluation of the association between genetic variants in circadian rhythm genes and posttraumatic stress symptoms identifies a potential functional allele in the transcription factor. Front Psychiatry. 2018;9:597.
Myers AJ. The age of the “ome”: genome, transcriptome and proteome data set collection and analysis. Brain Res Bull. 2012;88:294–301.
Myers AJ. AD gene 3-D: moving past single layer genetic information to map novel loci involved in Alzheimer’s disease. J Alzheimer’s Dis. 2013;33:S15–22.
Myers AJ. The genetics of gene expression multiple layers and multiple players. In: Coppola G, editor. The OMICs applications in neuroscience. New York, NY: Oxford University Press; 2014, pp 132–52.
Myers AJ, Gibbs JR, Webster JA, Rohrer K, Zhao A, Marlowe L, et al. A survey of genetic human cortical gene expression. Nat Genet. 2007;39:1494–9.
Webster JA, Gibbs JR, Clarke J, Ray M, Zhang W, Holmans P, et al. Genetic control of human brain transcript expression in Alzheimer disease. Am J Hum Genet. 2009;84:445–58.
Petyuk VA, Chang R, Ramirez-Restrepo M, Beckmann ND, Henrion MYR, Piehowski PD, et al. The human brainome: network analysis identifies HSPA2 as a novel Alzheimer’s disease target. Brain. 2020;141:2721–39.
Piehowski PD, Petyuk VA, Orton DJ, Xie F, Moore RJ, Ramirez-Restrepo M, et al. Sources of technical variability in quantitative LC-MS proteomics: human brain tissue sample analysis. J proteome Res. 2013;12:2128–37.
Lebron-Milad K, Graham BM, Milad MR. Low estradiol levels: a vulnerability factor for the development of posttraumatic stress disorder. Biol Psychiatry. 2012;72:6–7.
Milad MR, Zeidan MA, Contero A, Pitman RK, Klibanski A, Rauch SL, et al. The influence of gonadal hormones on conditioned fear extinction in healthy humans. Neuroscience. 2010;168:652–8.
Maddox SA, Kilaru V, Shin J, Jovanovic T, Almli LM, Dias BG, et al. Estrogen-dependent association of HDAC4 with fear in female mice and women with PTSD. Mol Psychiatry. 2018;23:658–65.
Elwood J, Murray E, Bell A, Sinclair M, Kernohan WG, Stockdale J. A systematic review investigating if genetic or epigenetic markers are associated with postnatal depression. J Affect Disord. 2019;253:51–62.
Chikahisa S, Chida D, Shiuchi T, Harada S, Shimizu N, Otsuka A, et al. Enhancement of fear learning in PPARalpha knockout mice. Behav Brain Res. 2019;359:664–70.
Maguschak KA, Ressler KJ. Wnt signaling in amygdala-dependent learning and memory. J Neurosci. 2011;31:13057–67.
Kim YA, Przytycki JH, Wuchty S, Przytycka TM. Modeling information flow in biological networks. Phys Biol. 2011;8:035012.
Kim YA, Wuchty S, Przytycka TM. Identifying causal genes and dysregulated pathways in complex diseases. PLoS Comput Biol. 2011;7:e1001095.
Shabalin AA. Matrix eQTL: ultra fast eQTL analysis via large matrix operations. Bioinformatics. 2012;28:1353–8.
Cerami EG, Gross BE, Demir E, Rodchenkov I, Babur O, Anwar N, et al. Pathway Commons, a web resource for biological pathway data. Nucleic Acids Res. 2011;39:D685–90.
Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Statist Soc B. 1995;57:289–300.
Wingo AP, Almli LM, Stevens JS, Stevens JJ, Klengel T, Uddin M, et al. DICER1 and microRNA regulation in post-traumatic stress disorder with comorbid depression. Nat Commun. 2015;6:10106.
Mercer KB, Dias B, Shafer D, Maddox SA, Mulle JG, Hu P, et al. Functional evaluation of a PTSD-associated genetic variant: estradiol regulation and ADCYAP1R1. Transl Psychiatry. 2016;6:e978.
Ressler KJ, Mercer KB, Bradley B, Jovanovic T, Mahan A, Kerley K, et al. Post-traumatic stress disorder is associated with PACAP and the PAC1 receptor. Nature. 2011;470:492–7.
Glover EM, Jovanovic T, Mercer KB, Kerley K, Bradley B, Ressler KJ, et al. Estrogen levels are associated with extinction deficits in women with posttraumatic stress disorder. Biol Psychiatry. 2012;72:19–24.
Graham BM, Milad MR. Blockade of estrogen by hormonal contraceptives impairs fear extinction in female rats and women. Biol Psychiatry. 2013;73:371–8.
Cornil CA, Seredynski AL, de Bournonville C, Dickens MJ, Charlier TD, Ball GF, et al. Rapid control of reproductive behaviour by locally synthesised oestrogens: focus on aromatase. J Neuroendocrinol. 2013;25:1070–8.
Gillespie CF, Almli LM, Smith AK, Bradley B, Kerley K, Crain DF, et al. Sex dependent influence of a functional polymorphism in steroid 5-alpha-reductase type 2 (SRD5A2) on post-traumatic stress symptoms. Am J Med Genet B Neuropsychiatr Genet. 2013;162B:283–92.
Fenchel D, Levkovitz Y, Vainer E, Kaplan Z, Zohar J, Cohen H. Beyond the HPA-axis: The role of the gonadal steroid hormone receptors in modulating stress-related responses in an animal model of PTSD. Eur Neuropsychopharmacol. 2015;25:944–57.
McEvoy K, Osborne LM, Nanavati J, Payne JL. Reproductive affective disorders: a review of the genetic evidence for premenstrual dysphoric disorder and postpartum depression. Curr Psychiatry Rep. 2017;19:94.
Gao L, Tober J, Gao P, Chen C, Tan K, Speck NA. RUNX1 and the endothelial origin of blood. Exp Hematol. 2018;68:2–9.
Scheitz CJ, Tumbar T. New insights into the role of Runx1 in epithelial stem cell biology and pathology. J Cell Biochem. 2013;114:985–93.
Wang JW, Stifani S. Roles of Runx genes in nervous system development. Adv Exp Med Biol. 2017;962:103–16.
Maguschak KA, Ressler KJ. Beta-catenin is required for memory consolidation. Nat Neurosci. 2008;11:1319–26.
Dias BG, Goodman JV, Ahluwalia R, Easton AE, Andero R, Ressler KJ. Amygdala-dependent fear memory consolidation via miR-34a and Notch signaling. Neuron. 2014;83:906–18.
Hong F, Pan S, Guo Y, Xu P, Zhai Y. PPARs as nuclear receptors for nutrient and energy metabolism. Molecules. 2019;24:2545.
Bordet R, Ouk T, Petrault O, Gelé P, Gautier S, Laprais M, et al. PPAR: a new pharmacological target for neuroprotection in stroke and neurodegenerative diseases. Biochem Soc Trans. 2006;34:1341–6.
Luo R, Su LY, Li G, Yang J, Liu Q, Yang LX, et al. Activation of PPARA-mediated autophagy reduces Alzheimer disease-like pathology and cognitive decline in a murine model. Autophagy. 2020;16:52–69.
Patel D, Roy A, Kundu M, Jana M, Luan CH, Gonzalez FJ, et al. Aspirin binds to PPARα to stimulate hippocampal plasticity and protect memory. Proc Natl Acad Sci USA. 2018;115:E7408–17.
Roy A, Jana M, Kundu M, Corbett GT, Rangaswamy SB, Mishra RK, et al. HMG-CoA reductase inhibitors bind to PPARα to upregulate neurotrophin expression in the brain and improve memory in mice. Cell Metab. 2015;22:253–65.
Foa EB, Riggs DS, Dancu CV, Rothbaum BO. Reliability and validity of a brief instrument for assessing post‐traumatic stress disorder. J Trauma Stress. 1993;6:459–73.
Foa EB, Tolin DF. Comparison of the PTSD symptom scale-interview version and the clinician-administered PTSD scale. J Trauma Stress. 2000;13:181–91.
Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–71.
Foa EB, Rothbaum BA. Treating the trauma of rape: cognitive behavioral therapy for PTSD. New York, NY: Guilford Press; 1998.
Rothbaum BO, Kearns MC, Price M, Malcoun E, Davis M, Ressler KJ, et al. Early intervention may prevent the development of posttraumatic stress disorder: a randomized pilot civilian study with modified prolonged exposure. Biol Psychiatry. 2012;72:957–63.
Rothbaum BO, Kearns MC, Reiser E, Davis JS, Kerley KA, Rothbaum AO, et al. Early intervention following trauma may mitigate genetic risk for PTSD in civilians: a pilot prospective emergency department study. J Clin Psychiatry. 2014;75:1380–7.
Kearns MC, Ressler KJ, Zatzick D, Rothbaum BO. Early interventions for PTSD: a review. Depress Anxiety. 2012;29:833–42.
Bernstein DP, Fink L, Handelsman L, Foote J, Lovejoy M, Wenzel K, et al. Initial reliability and validity of a new retrospective measure of child abuse and neglect. Am J Psychiatry. 1994;151:1132–6.
Michopoulos V, Beurel E, Gould F, Dhabhar FS, Schultebraucks K, Galatzer-Levy I, et al. Association of prospective risk for chronic PTSD symptoms with low TNFα and IFNγ concentrations in the immediate aftermath of trauma exposure. Am J Psychiatry. 2020;177:58–65.
Hinrichs R, van Rooij SJ, Michopoulos V, Schultebraucks K, Winters S, Maples-Keller J, et al. Increased skin conductance response in the immediate aftermath of trauma predicts PTSD risk. Chronic Stress. 2019;3:1–11.
Stevens JS, Kim YJ, Galatzer-Levy IR, Reddy R, Ely TD, Nemeroff CB, et al. Amygdala reactivity and anterior cingulate habituation predict posttraumatic stress disorder symptom maintenance after acute civilian trauma. Biol Psychiatry. 2017;81:1023–9.
Webster JA, Gibbs JR, Clarke J, Ray M, Zhang W, Holmans P, et al. Genetic control of human brain transcript expression in Alzheimer disease. Am J Hum Genet. 2009;84:445–58.
Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–75.
Anderson CA, Pettersson FH, Clarke GM, Cardon LR, Morris AP, Zondervan KT. Data quality control in genetic case-control association studies. Nat Protoc. 2010;5:1564–73.
Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet. 2006;38:904–9.
Delaneau O, Marchini J, Zagury JF. A linear complexity phasing method for thousands of genomes. Nat Methods. 2011;9:179–81.
Howie BN, Donnelly P, Marchini J. A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS Genet. 2009;5:e1000529.
Genomes Project C, Auton A, Brooks LD, Durbin RM, Garrison EP, Kang HM, et al. A global reference for human genetic variation. Nature. 2015;526:68–74.
Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21.
Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550.
Hoffman GE, Schadt EE. variancePartition: interpreting drivers of variation in complex gene expression studies. BMC Bioinforma. 2016;17:483.
Kanehisa M, Goto S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28:27–30.
Fabregat A, Jupe S, Matthews L, Sidiropoulos K, Gillespie M, Garapati P, et al. The reactome pathway knowledgebase. Nucleic Acids Res. 2018;46:D649–55.
Schaefer CF, Anthony K, Krupa S, Buchoff J, Day M, Hannay T, et al. PID: the Pathway Interaction Database. Nucleic Acids Res. 2009;37:D674–9.
Gandhi TK, Zhong J, Mathivanan S, Karthick L, Chandrika KN, Mohan SS, et al. Analysis of the human protein interactome and comparison with yeast, worm and fly interaction datasets. Nat Genet. 2006;38:285–93.
Stark C, Breitkreutz BJ, Reguly T, Boucher L, Breitkreutz A, Tyers M. BioGRID: a general repository for interaction datasets. Nucleic Acids Res. 2006;34:D535–9.
Bader GD, Betel D, Hogue CW. BIND: the Biomolecular Interaction Network Database. Nucleic Acids Res. 2003;31:248–50.
Bian Y, Yang L, Zhao M, Li Z, Xu Y, Zhou G, et al. Identification of key genes and pathways in post-traumatic stress disorder using microarray analysis. Front Psychol. 2019;10:302.
Xu N, Zhou WJ, Wang Y, Huang SH, Li X, Chen ZY. Hippocampal Wnt3a is necessary and sufficient for contextual fear memory acquisition and consolidation. Cereb Cortex. 2015;25:4062–75.
Jiang P, Zhang WY, Li HD, Cai HL, Liu YP, Chen LY. Stress and vitamin D: altered vitamin D metabolism in both the hippocampus and myocardium of chronic unpredictable mild stress exposed rats. Psychoneuroendocrinology. 2013;38:2091–8.
Ji LL, Tong L, Peng JB, Jin XH, Wei D, Xu BK, et al. Changes in the expression of the vitamin D receptor and LVSCC‑A1C in the rat hippocampus submitted to single prolonged stress. Mol Med Rep. 2014;9:1165–70.
Kuningas M, Mooijaart SP, Jolles J, Slagboom PE, Westendorp RG, van Heemst D. VDR gene variants associate with cognitive function and depressive symptoms in old age. Neurobiol Aging. 2009;30:466–73.
Kéri S, Szabó C, Kelemen O. Blood biomarkers of depression track clinical changes during cognitive-behavioral therapy. J Affect Disord. 2014;164:118–22.
Muminovic Umihanic M, Babic R, Kravic N, Avdibegovic E, Dzubur Kulenovic A, Agani F, et al. Associations between polymorphisms in the solute carrier family 6 member 3 and the myelin basic protein gene and posttraumatic stress disorder. Psychiatr Danub. 2019;31:235–40.
Wang Q, Wang Z, Zhu P, Jiang J. Alterations of myelin basic protein and ultrastructure in the limbic system at the early stage of trauma-related stress disorder in dogs. J Trauma. 2004;56:604–10.
Han Y, Sun CY, Meng SQ, Tabarak S, Yuan K, Cao L, et al. Systemic immunization with altered myelin basic protein peptide produces sustained antidepressant-like effects. Mol Psychiatry. 2020;25:1260–74.
Lowe SR, Meyers JL, Galea S, Aiello AE, Uddin M, Wildman DE, et al. RORA and posttraumatic stress trajectories: main effects and interactions with childhood physical abuse history. Brain Behav. 2015;5:e00323.
Miller MW, Wolf EJ, Logue MW, Baldwin CT. The retinoid-related orphan receptor alpha (RORA) gene and fear-related psychopathology. J Affect Disord. 2013;151:702–8.
Amstadter AB, Sumner JA, Acierno R, Ruggiero KJ, Koenen KC, Kilpatrick DG, et al. Support for association of RORA variant and post traumatic stress symptoms in a population-based study of hurricane exposed adults. Mol Psychiatry. 2013;18:1148–9.
Logue MW, Baldwin C, Guffanti G, Melista E, Wolf EJ, Reardon AF, et al. A genome-wide association study of post-traumatic stress disorder identifies the retinoid-related orphan receptor alpha (RORA) gene as a significant risk locus. Mol Psychiatry. 2013;18:937–42.
Uddin M, Ratanatharathorn A, Armstrong D, Kuan PF, Aiello AE, Bromet EJ, et al. Epigenetic meta-analysis across three civilian cohorts identifies NRG1 and HGS as blood-based biomarkers for post-traumatic stress disorder. Epigenomics. 2018;10:1585–601.
Chen YH, Lan YJ, Zhang SR, Li WP, Luo ZY, Lin S, et al. ErbB4 signaling in the prelimbic cortex regulates fear expression. Transl Psychiatry. 2017;7:e1168.
Lu Y, Sun XD, Hou FQ, Bi LL, Yin DM, Liu F, et al. Maintenance of GABAergic activity by neuregulin 1-ErbB4 in amygdala for fear memory. Neuron. 2014;84:835–46.
Taylor SB, Taylor AR, Koenig JI. The interaction of disrupted type II neuregulin 1 and chronic adolescent stress on adult anxiety- and fear-related behaviors. Neuroscience. 2013;249:31–42.
Kilaru V, Iyer SV, Almli LM, Stevens JS, Lori A, Jovanovic T, et al. Genome-wide gene-based analysis suggests an association between Neuroligin 1 (NLGN1) and post-traumatic stress disorder. Transl Psychiatry. 2016;6:e820.
Pomytkin I, Costa-Nunes JP, Kasatkin V, Veniaminova E, Demchenko A, Lyundup A, et al. Insulin receptor in the brain: mechanisms of activation and the role in the CNS pathology and treatment. CNS Neurosci Ther. 2018;24:763–74.
Bigio B, Mathé AA, Sousa VC, Zelli D, Svenningsson P, McEwen BS, et al. Epigenetics and energetics in ventral hippocampus mediate rapid antidepressant action: Implications for treatment resistance. Proc Natl Acad Sci USA. 2016;113:7906–11.
Sakharkar AJ, Zhang H, Tang L, Baxstrom K, Shi G, Moonat S, et al. Effects of histone deacetylase inhibitors on amygdaloid histone acetylation and neuropeptide Y expression: a role in anxiety-like and alcohol-drinking behaviours. Int J Neuropsychopharmacol. 2014;17:1207–20.
Lu HC, Tan Q, Rousseaux MW, Wang W, Kim JY, Richman R, et al. Disruption of the ATXN1-CIC complex causes a spectrum of neurobehavioral phenotypes in mice and humans. Nat Genet. 2017;49:527–36.
Liu J, Su B. Integrated analysis supports ATXN1 as a schizophrenia risk gene. Schizophr Res. 2018;195:298–305.
Funding
This work was supported by the National Institute on Aging (AG063688), the Howard Hughes Medical Institute, the National Institute for Health (R01 MH094757, KJR; R01 MH094759, CBN), the National Institute of Child Health and Human Development (K12 HD085850, VM), and the Brain and Behavior Research Foundation (BOR). Additional support was provided by the National Center for Advancing Translational Sciences of the NIH under award UL1TR002378.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
KJR provides fee-for-service consultation for Johnson & Johnson, Verily, and Alkermes. He has received sponsored research unrelated to this work from Brainsway and Takeda. He also holds patents for a number of targets related to improving extinction of fear; however, he has received no equity or income within the last 3 years related to these. He receives or has received research funding from NIMH, NIAAA, HHMI, NARSAD, and the Burroughs Wellcome Foundation.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wuchty, S., Myers, A.J., Ramirez-Restrepo, M. et al. Integration of peripheral transcriptomics, genomics, and interactomics following trauma identifies causal genes for symptoms of post-traumatic stress and major depression. Mol Psychiatry 26, 3077–3092 (2021). https://doi.org/10.1038/s41380-021-01084-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-021-01084-3
This article is cited by
-
Molecular pathways of major depressive disorder converge on the synapse
Molecular Psychiatry (2023)
-
A novel circular RNA, circIgfbp2, links neural plasticity and anxiety through targeting mitochondrial dysfunction and oxidative stress-induced synapse dysfunction after traumatic brain injury
Molecular Psychiatry (2022)
-
Post-traumatic stress disorder: clinical and translational neuroscience from cells to circuits
Nature Reviews Neurology (2022)
-
Amygdala DCX and blood Cdk14 are implicated as cross-species indicators of individual differences in fear, extinction, and resilience to trauma exposure
Molecular Psychiatry (2022)