Abstract
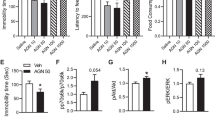
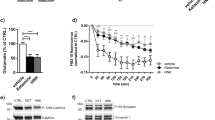
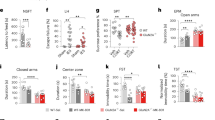
Both the NMDA receptor (NMDAR) positive allosteric modulator (PAM), and antagonist, can exert rapid antidepressant effects as shown in several animal and human studies. However, how this bidirectional modulation of NMDARs causes similar antidepressant effects remains unknown. Notably, the initial cellular trigger, specific cell-type(s), and subunit(s) of NMDARs mediating the antidepressant-like effects of a PAM or an antagonist have not been identified. Here, we used electrophysiology, microdialysis, and NMR spectroscopy to evaluate the effect of a NMDAR PAM (rapastinel) or NMDAR antagonist, ketamine on NMDAR function and disinhibition-mediated glutamate release. Further, we used cell-type specific knockdown (KD), pharmacological, and behavioral approaches to dissect the cell-type specific role of GluN2B, GluN2A, and dopamine receptor subunits in the actions of NMDAR PAM vs. antagonists. We demonstrate that rapastinel directly enhances NMDAR activity on principal glutamatergic neurons in medial prefrontal cortex (mPFC) without any effect on glutamate efflux, while ketamine blocks NMDAR on GABA interneurons to cause glutamate efflux and indirect activation of excitatory synapses. Behavioral studies using cell-type-specific KD in mPFC demonstrate that NMDAR-GluN2B KD on Camk2a- but not Gad1-expressing neurons blocks the antidepressant effects of rapastinel. In contrast, GluN2B KD on Gad1- but not Camk2a-expressing neurons blocks the actions of ketamine. The results also demonstrate that Drd1-expressing pyramidal neurons in mPFC mediate the rapid antidepressant actions of ketamine and rapastinel. Together, these results demonstrate unique initial cellular triggers as well as converging effects on Drd1-pyramidal cell signaling that underlie the antidepressant actions of NMDAR-positive modulation vs. NMDAR blockade.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Hasin DS, Sarvet AL, Meyers JL, Saha TD, Ruan WJ, Stohl M, et al. Epidemiology of adult DSM-5 major depressive disorder and Its specifiers in the United States. JAMA Psychiatry. 2018;75:336–46.
Fava M. Diagnosis and definition of treatment-resistant depression. Biol Psychiatry. 2003;53:649–59.
Trivedi M, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, STAR*D Study Team, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163:28–40.
Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–4.
Abdallah CG, Sanacora G, Duman RS, Krystal JH. Ketamine and rapid-acting antidepressants: a window into a new neurobiology for mood disorder therapeutics. Annu Rev Med. 2015;66:509–23.
Sleigh J, Harvey M, Voss L, Denny B. Ketamine—more mechanisms of action than just NMDA blockade. Trends Anaesth Crit Care. 2014;4:76–81.
Morgan CJA, Curran HV. Ketamine use: a review. Addiction. 2012;107:27–38.
Moskal JR, Burch R, Burgdorf JS, Kroes RA, Stanton PK, Disterhoft JF, et al. GLYX-13, an NMDA receptor glycine site functional partial agonist enhances cognition and produces antidepressant effects without the psychotomimetic side effects of NMDA receptor antagonists. Expert Opin Investig Drugs. 2014;23:243–54.
Burch R, Preskorn S, Bastin L, Yu W, Burgdorf J, Moskal J. Adjunctive GLYX-13 induces prolonged efficacy in subjects with major depressive disorder (MDD). Neuropsychopharmacology. 2014;39:S335.
Preskorn S, Macaluso M, Mehra DO, Zammit G, Moskal JR, Burch RM, et al. Randomized proof of concept trial of GLYX-13, an N-methyl-D-aspartate receptor glycine site partial agonist, in major depressive disorder nonresponsive to a previous antidepressant agent. J Psychiatr Pract. 2015;21:140–9.
Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–64.
Liu RJ, Duman C, Kato T, Hare B, Lopresto D, Bang E, et al. GLYX-13 produces rapid antidepressant responses with key synaptic and behavioral effects distinct from ketamine. Neuropsychopharmacology. 2017;42:1231–42.
Kato T, Fogaca M, Deyama S, Li X, Fukumoto K, Duman R. BDNF release and signaling are required for the antidepressant actions of GLYX-13. Mol Psych. 2017;23:2007–17.
Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17:2921–7.
Lorrain DS, Baccei CS, Bristow LJ, Anderson JJ, Varney MA. Effects of ketamine and N-methyl-D-aspartate on glutamate and dopamine release in the rat prefrontal cortex: modulation by a group II selective metabotropic glutamate receptor agonist LY379268. Neuroscience. 2003;117:697–706.
Homayoun H, Moghaddam B. NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J Neurosci. 2007;27:11496–500.
Gerhard D, Pothula S, Liu RJ, Wu M, Li XY, Girgenti MJ, et al. GABA interneurons are the initial trigger for the rapid antidepressant actions of ketamine. J Clin Invest. 2020;130:1336–49.
Preskorn S, Baker B, Kolluri S, Menniti FS, Krams M, Landen JW. An innovative design to establish proof of concept of the antidepressant effects of the NR2B subunit selective N-Methyl-D-Aspartate antagonist, CP-101,606, in patients with treatment-refractory major depressive disorder. J Clin Psychopharmacol. 2008;28:631–7.
Yang CR, Seamans JK, Gorelova N. Electrophysiological and morphological properties of layers V-VI principal pyramidal cells in rat prefrontal cortex in vitro. J Neurosci. 1996;16:1904–21.
Santana N, Mengod G, Artigas F. Quantitative analysis of the expression of dopamine D1 and D2 receptors in pyramidal and GABAergic neurons of the rat prefrontal cortex. Cereb Cortex. 2009;19:849–60.
Wei X, Ma T, Cheng Y, Huang CCY, Wang X, Lu J, et al. Dopamine D1 or D2 receptor-expressing neurons in the central nervous system. Addict Biol. 2018;23:569–84.
Anastasiades PG, Boada C, Carter AG. Cell-Type-Specific D1 dopamine receptor modulation of projection neurons and interneurons in the prefrontal cortex. Cereb Cortex 2018;29:3224–42.
Gee S, Ellwood I, Patel T, Luongo F, Deisseroth K, Sohal VS. Synaptic activity unmasks dopamine D2 receptor modulation of a specific class of layer V pyramidal neurons in prefrontal cortex. J Neurosci. 2012;32:4959–71.
Kehr J, Yoshitake T. Monitoring brain chemical signals by microdialysis. In: Grimes CA, Dickey EC, Pishko MV, editors. Encyclopedia of sensors, Vol. 6. USA: American Scientific Publishers; 2006. p. 287–312.
Kehr J, Yoshitake T. Derivatization chemistries for improved detection of monoamine neurotransmitters and their metabolites in microdialysis samples by liquid chromatography with fluorescence detection and mass spectrometry. In: Wilson G, Michael A, editors. Compendium of in-vivo monitoring in real-time molecular neuroscience, Vol. 2, microdialysis and sensing of neural tissues. Singapore: World Scientific Publishing; 2017. p. 193–216.
Chowdhury GM, Behar KL, Cho W, Thomas MA, Rothman DL, Sanacora G. (1)H-[(1)(3)C]-nuclear magnetic resonance spectroscopy measures of ketamine’s effect on amino acid neurotransmitter metabolism. Biolo Psychiatry. 2012;71:1022–25.
Chowdhury GM, Zhang J, Thomas M, et al. Transiently increased glutamate cycling in rat PFC is associated with rapid onset of antidepressant-like effects. Mol Psychiatry. 2017;22:120–6.
Shinohara R, Taniguchi M, Ehrlich AT, Yokogawa K, Deguchi Y, Cherasse Y, et al. Dopamine D1 receptor subtype mediates acute stress-induced dendritic growth in excitatory neurons of the medial prefrontal cortex and contributes to suppression of stress susceptibility in mice. Mol Psychiatry. 2018;23:1717–30.
Wohleb ES, Wu M, Gerhard DM, Taylor SR, Picciotto MR, Alreja M, et al. GABA interneurons mediate the rapid antidepressant-like effects of scopolamine. J Clin Invest. 2016;126:2482–94.
Duman RS, Shinohara R, Fogaça MV, Hare B. Neurobiology of rapid-acting antidepressants: convergent effects on GluA1-synaptic function. Mol Psychiatry. 2019;24:1816–32.
White PF, Schüttler J, Shafer A, Stanski DR, Horai Y, Trevor AJ. Comparative pharmacology of the ketamine isomers: studies in volunteers. Br J Anaesth. 1985;57:197–203.
Donello JE, Banerjee P, Li XY, Guo YX, Yoshitake T, Zhang XL, et al. Positive N-Methyl-D-Aspartate receptor modulation by rapastinel promotes rapid and sustained antidepressant-like effects. Int J Neuropsychopharmacol. 2019;22:247–59.
Burgdorf J, Zhang XL, Nicholson KL, Balster RL, Leander JD, Stanton PK, et al. GLYX-13, an NMDA receptor glycine-site functional partial agonist, induces antidepressant-like effects without ketamine-like side effects. Neuropsychopharmacology. 2013;38:729–42.
Fuchikami M, Thomas A, Liu R, Wohleb ES, Land BB, DiLeone RJ, et al. Optogenetic stimulation of infralimbic PFC reproduces ketamine’s rapid and sustained antidepressant actions. Proc Natl Acad Sci USA. 2015;112:8106–11.
Tanaka K, Furuyashiki T, Kitaoka S, Senzai Y, Imoto Y, Segi-Nishida E, et al. Prostaglandin E2-mediated attenuation of mesocortical dopaminergic pathway is critical for susceptibility to repeated social defeat stress in mice. J Neurosci. 2012;32:4319–29.
Anderson EM, Gomez D, Caccamise A, McPhail D, Hearing M. Chronic unpredictable stress promotes cell-specific plasticity in prefrontal cortex D1 and D2 pyramidal neurons. Neurobiol Stress. 2019;10:100152.
Hare BD, Shinohara R, Liu RJ, Pothula S, DiLeone RJ, Duman RS. Optogenetic stimulation of medial prefrontal cortex Drd1 neurons produces rapid and long-lasting antidepressant effects. Nat Commun. 2019;10:223.
Rajagopal L, Huang M, Li J, He W, Soni D, Banerjee P, et al. Rapastinel, a novel NMDA receptor modulator, produces prolonged rescue of subchronic phencyclidine-induced deficits in episodic memory as well as other beneficial effects on cognitive function in a rapamycin sensitive manner. 341.12 / VV3. 2017 Neuroscience Meeting Planner. Washington, DC: Society for Neuroscience; 2017.
Widman AJ, McMahon LL. Disinhibition of CA1 pyramidal cells by low-dose ketamine and other antagonists with rapid antidepressant efficacy. Proc Natl Acad Sci USA. 2018;115:E3007–16.
Miller OH, Yang L, Wang CC, Hargroder EA, Zhang Y, Delpire E. et al. GluN2B-containing NMDA receptors regulate depression-like behavior and are critical for the rapid antidepressant actions of ketamine. Elife. 2014;3:e03581. https://doi.org/10.7554/eLife.03581.
Miller OH, Bruns A, Ben Ammar I, Mueggler T, Hall BJ. Synaptic regulation of a thalamocortical circuit controls depression-related behavior. Cell Rep. 2017;20:1867–80.
Feyissa AM, Chandran A, Stockmeier CA, Karolewicz B. Reduced levels of NR2A and NR2B subunits of NMDA receptor and PSD-95 in the prefrontal cortex in major depression. Prog Neuro-Psychopharmacol Biol Psychiatry. 2009;33:70–5.
Nowak G, Ordway GA, Paul IA. Alterations in the N-methyl-d-asparatate (NMDA) receptor complex in the frontal cortex of suicide victims. Brain Res. 1995;675:157–64.
Land BB, Narayanan NS, Liu RJ, Gianessi CA, Brayton CE, Grimaldi D, et al. Medial prefrontal D1 dopamine neurons control food intake. Nat Neurosci. 2014;17:248–53.
Chaudhury D, Walsh JJ, Friedman AK, Juarez B, Ku SM, Koo JW, et al. Rapid regulation of depression-related behaviors by control of midbrain dopamine neurons. Nature. 2013;493:532–6.
Gao C, Wolf ME. Dopamine receptors regulate NMDA receptor surface expression in prefrontal cortex neurons. J Neurochem. 2008;106:2489–501.
Sun X, Zhao Y, Wolf ME. Dopamine receptor stimulation modulates AMPA receptor synaptic insertion in prefrontal cortex neurons. J Neurosci. 2005;25:7342–51.
Lavin A, Grace AA. Stimulation of D1-type dopamine receptors enhances excitability in prefrontal cortical pyramidal neurons in a state-dependent manner. Neuroscience. 2001;104:335–46.
Gurden H, Takita M, Jay TM. Essential role of D1 but not D2 receptors in the NMDA receptor‐dependent long‐term potentiation at hippocampal‐prefrontal cortex synapses in vivo. J Neurosci. 2000;20:RC106.
Jay TM. Dopamine: a potential substrate for synaptic plasticity and memory mechanisms. Prog Neurobiol. 2003;69:375–90.
Berry A, Bellisario V, Capoccia S, Tirassa P, Calza A, Alleva E, et al. Social deprivation stress is a triggering factor for the emergence of anxiety- and depression-like behaviours and leads to reduced brain BDNF levels in C57BL/6J mice. Psychoneuroendocrinology. 2012;37:762–72.
Ieraci A, Mallei A, Popoli M. Social isolation stress induces anxious-depressive-like behavior and alterations of neuroplasticity-related genes in adult male mice. Neural Plast. 2016;2016:6212983.
Mumtaz F, Khan MI, Zubair M, Dehpour AR. Neurobiology and consequences of social isolation stress in animal model-A comprehensive review. Biomed Pharmacother. 2018;105:1205–22.
Haj-Mirzaian A, Amini-Khoei H, Haj-Mirzaian A, Amiri S, Ghesmati M, Zahir M, et al. Activation of cannabinoid receptors elicits antidepressant-like effects in a mouse model of social isolation stress. Brain Res Bull. 2017;130:200–10.
Acknowledgements
This research was supported by National Institute of Mental Health grants MH093897 and MH105910 (RSD) and a research grant from Allergan Inc. New Jersey. We thank Xiao Yuan Li for her help with genotyping of mouse lines. We thank Jan Kehr (Pronexus Analytical AB, Stockholm, Sweden) for performing the microdialysis experiments. Dr. Duman passed away on February 1st 2020. This article is dedicated to Dr. Duman in memory of his mentorship and scientific leadership.
Author information
Authors and Affiliations
Contributions
SP, TK, PB, and RSD designed the study. SP wrote the manuscript and RSD contributed to writing the manuscript. SP, TK, RJL, and MW conducted experiments, analyzed data, and interpreted the results. GS, GMIC, and KLB designed glutamate cycling experiment using MRS spectroscopy. GMIC and KLB conducted glutamate cycling experiment. DG and RS are involved in design and preparation of pGluN2BshRNA (dsRed version) and AAV-DIO-EmGFP-D1miR viruses. A-NS helped with experiments. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
RSD has received consulting fees from Taisho, Johnson & Johnson, and Naurex, and grant support from Taisho, Johnson & Johnson, Naurex, Allergan, Navitor, Lundbeck, Relmada, and Lilly. PB is an employee of Allergan Inc, New Jersey. All other authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Pothula, S., Kato, T., Liu, RJ. et al. Cell-type specific modulation of NMDA receptors triggers antidepressant actions. Mol Psychiatry 26, 5097–5111 (2021). https://doi.org/10.1038/s41380-020-0796-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-020-0796-3
This article is cited by
-
Ketamine and rapid antidepressant action: new treatments and novel synaptic signaling mechanisms
Neuropsychopharmacology (2024)
-
Targeting metaplasticity mechanisms to promote sustained antidepressant actions
Molecular Psychiatry (2024)
-
The Role of Extrasynaptic GABA Receptors in Postpartum Depression
Molecular Neurobiology (2024)
-
M1 acetylcholine receptors in somatostatin interneurons contribute to GABAergic and glutamatergic plasticity in the mPFC and antidepressant-like responses
Neuropsychopharmacology (2023)
-
Central medial thalamic nucleus dynamically participates in acute itch sensation and chronic itch-induced anxiety-like behavior in male mice
Nature Communications (2023)